Approximately 48,960 people in the USA will be diagnosed with pancreatic cancer in 2015 and 40,560 will die for this reason; in Mexico, the new cases of pancreatic cancer in 2012 were 4274, with 4133 deaths; survival rate at 5 years goes from 1% to 15%. Less than 20% of cases were considered resectable at the time of diagnosis. The Whipple procedure is currently the only curative treatment option for periampullary cancers since the first communication by Whipple in 1935, and up until now is a common procedure in several reference centres around the world. In 1994, Gagner reported the first totally laparoscopic pancreaticoduodenectomy. Some groups have currently demonstrated the safety and efficacy of this technique.
ObjectiveTo report our initial experience with totally laparoscopic pancreaticoduodenectomy in the Hospital General de México.
Clinical caseThe case concerns a 58 year-old women with jaundice and loss of weight of 3 months onset. Her biopsy reported adenocarcinoma of Váter's ampulla, and as it was considered resectable, she underwent a laparoscopic pancreaticoduodenectomy.
ConclusionsThis procedure must be performed in centres with experience in open pancreatic surgery and training in advanced laparoscopic surgery. The main advantages are lower blood loss and shorter hospital stay.
Se estima que en el 2015 en Estados Unidos de Norteamérica 48,960 personas serán diagnosticadas con cáncer de páncreas y 40,560 morirán por esta causa; en México, el número de nuevos casos en 2012 fue de 4,274, mientras que se presentaron 4,133 defunciones; la sobrevida a 5 años va del 1 al 15%. Menos del 20% de los casos son considerados resecables al momento del diagnóstico. La cirugía de Whipple continúa siendo hoy en día la única opción de tratamiento con intento curativo para la enfermedad tumoral periampular, desde la primera publicación por Whipple en 1935 hasta nuestros días, en que se realiza con frecuencia en centros de referencia. En 1994, Gagner reportó la primera pancreatoduodenectomía realizada completamente por vía laparoscópica. Diversos grupos han demostrado la seguridad y eficacia del empleo de este abordaje.
ObjetivoComunicar nuestra experiencia inicial con la pancreatoduodenectomía totalmente laparoscópica en el Hospital General de México.
Caso clínicoSe reporta el caso de una mujer de 58 años con ictericia obstructiva y pérdida de peso de 3 meses de evolución, a quién se diagnostica adenocarcinoma de ámpula de Váter, se consideró resecable por estudios de imagen y se realizó cirugía de Whipple, por vía laparoscópica.
ConclusiónEste tipo de procedimiento debe ser realizado en centros con experiencia en cirugía pancreática abierta, con adiestramiento en cirugía laparoscópica avanzada. La ventaja de este abordaje se centra, principalmente, en un menor sangrado transoperatorio y menor estancia hospitalaria.
The American Cancer Society estimates that 48,960 people will be diagnosed with pancreatic cancer in 2015; 40,560 will die from this disease.1 In Mexico, according to the latest information from Globanc, there were 4274 new cases in 2012, and 4133 deaths.2 Survival at 5 years ranges from 1% to 15% according to the clinical stage at time of diagnosis. Somewhat fewer than one in every 5 cases are considered resectable at time of diagnosis.3
Pancreatoduodenectomy or Whipple's procedure, as it is also known, is still the only treatment option with curative intent for periampullary cancer,4 covering pancreatic adenocarcinoma, distal cholangiocarcinoma, ampullary adenocarcinoma and duodenal adenocarcinoma.
Over the years, advances in medical and particularly surgical techniques have resulted in the application of new surgical procedures and more and better technological tools in the area of pancreatic surgery.
Whipple's procedure has evolved since the first publication in 1935, when the resection was completed in 2 stages5 (it is described in one stage in 1941),6 with some referral centres currently reporting over 100 procedures a year.7,8 This evolution has resulted in an improved mortality rate at less than 2%. However, morbidity has remained constant at between 30% and 40%.
In 1994, Gagner reported the first pancreatoduodenectomy to be performed completely laparoscopically.9 Initially this was not well accepted because of the complexity, multiple anastomoses and length of the procedure.
Various groups have now demonstrated the safety and efficacy of the laparoscopic approach in pancreatoduodenectomy.10
The objective of this study was to make known our initial experience with a pancreatoduodenectomy performed entirely laparoscopic ally, and the surgical technique we used in the Hospital General de México.
Clinical caseA 58-year-old female patient, from the city of Guanajuato, and a housewife. With a history of type 2 diabetes mellitus of 12 years onset, managed initially by diet and then oral hypoglycaemic agents and 3 years prior to admission by insulin glargine, 7IU per day, with adequate control of the disease.
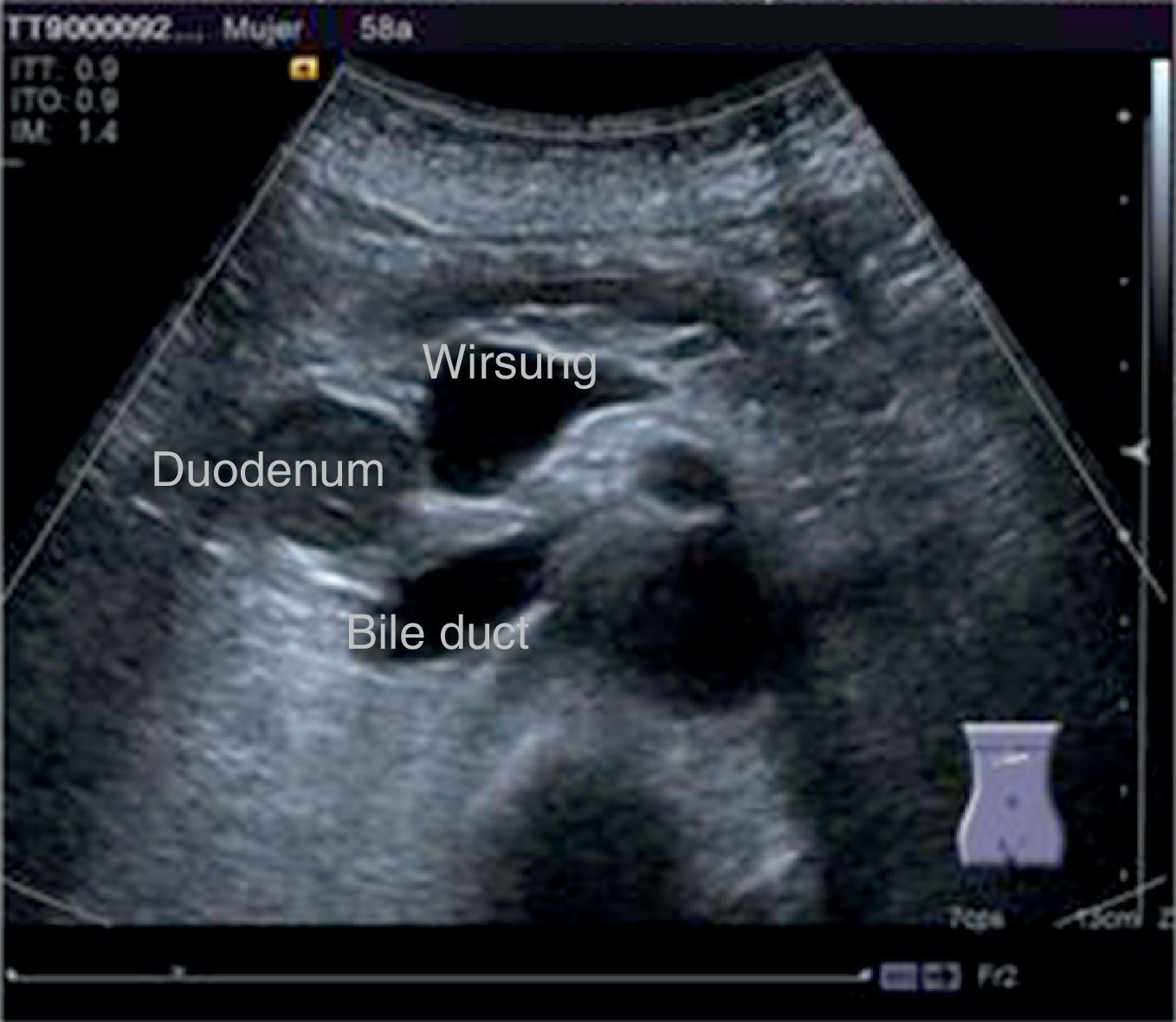
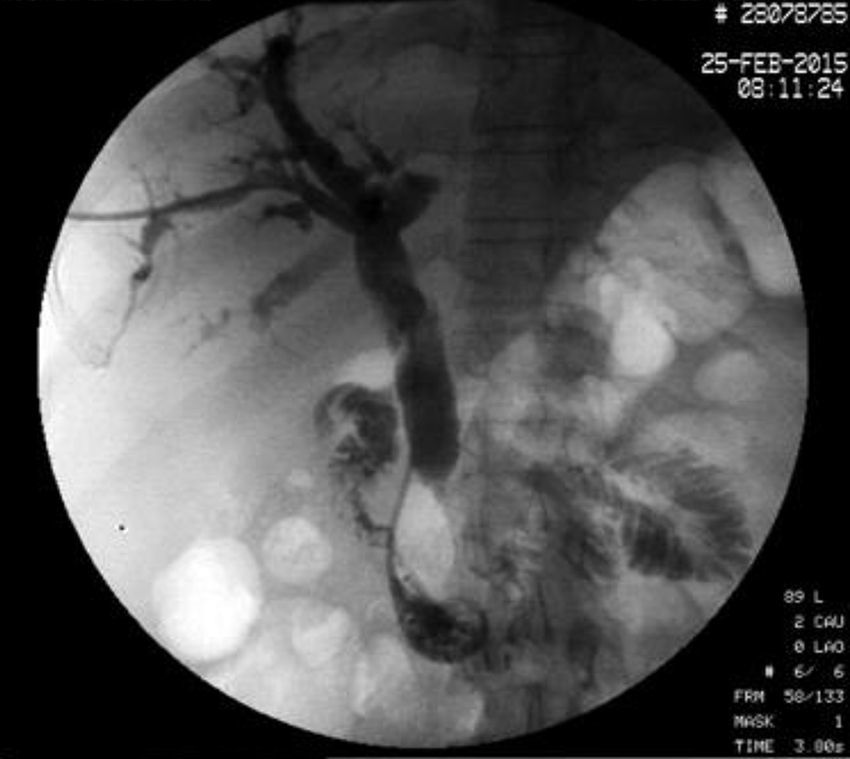
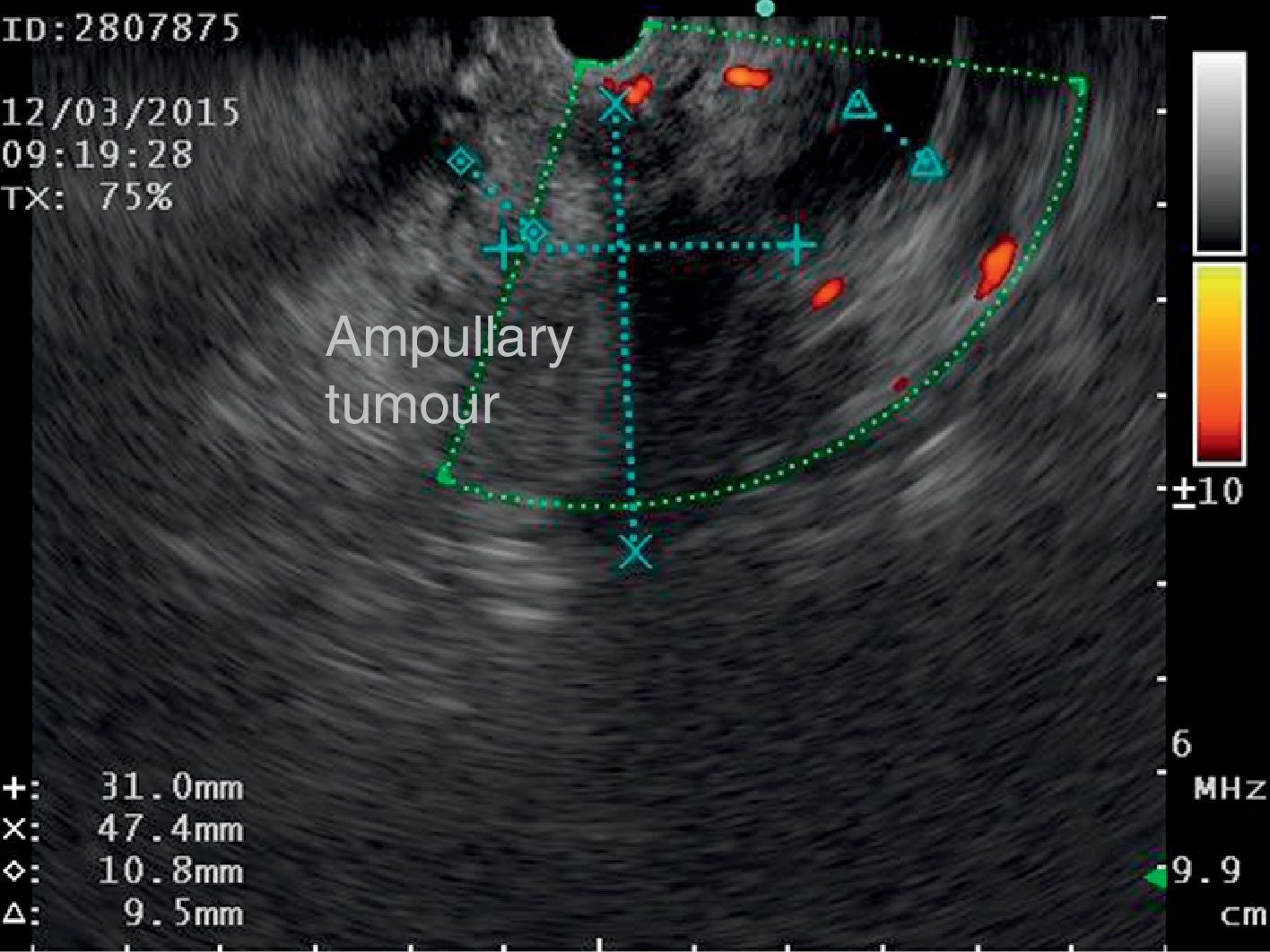
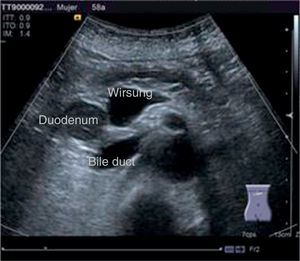
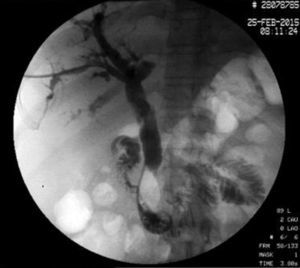
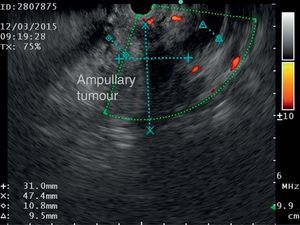
The disease started 3 months prior to her admission when the patient presented jaundice, choluria, asthenia, adynamia, with weight loss of approximately 10kg, over the same period. On admission she presented jaundiced skin and sclera. General examination showed no pathological signs. The laboratory tests reported total bilirrubin 15.5mg/dl, direct bilirrubin 8.9mg/dl, alkaline phosphatase 1860u/l, GGT 2217u/l, with albumin 1.4g/dl. Abdominal ultrasound reported dilation of the intra and extrahepatic bile duct, common bile duct 17.8mm, with hyperechoic images inside relating to biliary sludge. The bile duct had heterogeneous predominantly hyperechoic content due to biliary sludge. The pancreatic gland had a heterogeneous ultrasound pattern, duct of Wirsung 3.8mm, thickening in the second portion of the duodenum, related to a probable duodenal neoplasm (Fig. 1). Upper endoscopy showed no alterations in the stomach. It was not possible to pass the endoscope beyond the first portion of the duodenum due to stenosis of the lumen at this level, no ulcerated mucosa or areas showing tumour or infiltration. Percutaneous transhepatic cholangiography was performed, showing dilated bile ducts, with some segmentary dilatation associated with peri-cholangitic abscesses. During this procedure an 8.5 Fr catheter was placed, which was passed up to the duodenal lumen (Fig. 2). Axial computed tomography with pancreatic protocol showed an enlarged liver with left lobe predominance, homogeneous parenchyma and large dilation of the intrahepatic bile duct, and pancreas with homogeneous attenuation pattern. Wall thickening was observed in the duodenum that reduced its lumen by approximately 90%, which was enhanced by contrast medium, with a plane of separation with the pancreatic head, relating to a tumour of approximately 4cm of the ampulla of Vater. Endoscopic ultrasound was performed showing a lesion of 41mm×31mm in the pancreatic head, which extended towards the periampullary region and duodenal wall; the vascular structures were intact. No adenopathies were identified (Fig. 3). Aspiration biopsy reported moderately differentiated adenocarcinoma of the ampulla of Vater. Enteral support was decided after nutritional assessment to improve the patient's condition and she was scheduled for laparoscopic pancreatoduodenectomy on 1 June 2015.
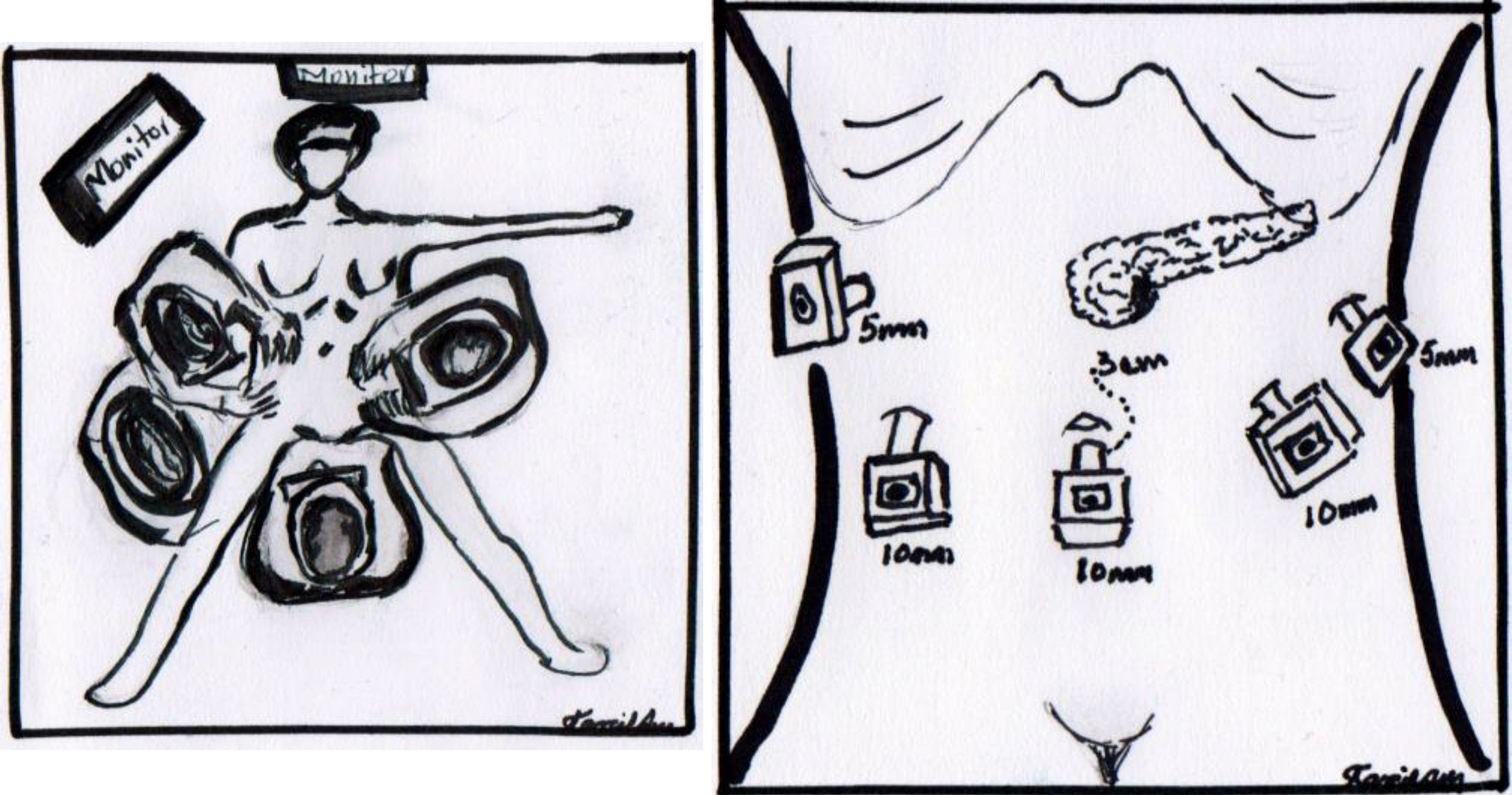
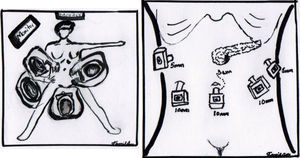
The operation was performed under balanced general anaesthetic, with the patient in a supine position, in the Lloyd-Davis position, with the surgeon on foot between her legs, and an assistant at each side. A pneumoperitoneum was created with a Veress needle inserted into the umbilical scar, with an average pressure of 12mm/Hg. A 10mm trocar was inserted through this site for the optic system. Two additional trocars were inserted at the level of the umbilical scar and its intersection with the left and right clavicular midline, a 5mm trocar at the level of the right clavicular midline 6cm above the former, for the first assistant, and a final 5mm trocar below the left ribcage border where it joins the anterior axillary line, for the second assistant (Fig. 4). The entire peritoneal cavity was explored with no finding of signs of distant metastases; it was therefore decided to evaluate resectability.
A harmonic scalpel and Enseal (both by Ethicon Endo-surgery©) were used for dissection and occlusion of blood vessels.
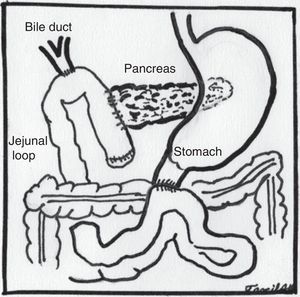
The sequence of stages was as follows: (1) the gastrocolic ligament was opened from where the right and left gastroepiploic arteries join up to the right gastric vein, entering the transcavity and uncovering the anterior pancreatic face. (2) The hepatocolic ligament was sectioned to lower the hepatic flexure of the colon using an Enseal. (3) The right gastroepiploic artery and the right gastric vein were ligated at the inner edge of the first portion of the duodenum. (4) The duodenum was divided at 4cm from the pylorus with Endo-GIA stapler (blue load). (5) Calot's triangle was dissected and the artery and cystic duct clipped and cut. (6) The hepatoduodenal ligament was dissected and elements of the hepatic pedicle, identifying the hepatic artery, gastroduodenal artery, which was dissected, titanium clips placed and cut. (7) The areolar and lymphatic tissue surrounding the bile duct was dissected and released. (8) The lower edge of the pancreas was dissected at the level of the pancreatic neck, separating mesenteric vessels and the portal vein through the posterior face, until reaching the upper border of the pancreas which was found with a ¼ Penrose. (9) The jejunum was sectioned at 15cm from the angle of Treitz with an Endo-GIA stapler (blue load), the Treitz ligament was disinserted and the jejunal loop passed to the right of the mesenteric vessels. (10) A section was made at the level of the pancreatic neck using a harmonic scalpel, identifying the main pancreatic duct, which was cut with cold scissors. (11) The common hepatic duct was completely cut above the insertion of the cystic duct. (12) A Kocher's manoeuvre was made up to the third portion of the duodenum and in its posterior edge until the vena cava and the lateral edge of the aortic artery were exposed to visualise the left renal vein. (13) The uncinate process was dissected and released, clipping and occluding the veins and arteries as necessary using a harmonic scalpel and staples as required. Resection of the surgical piece was completed and placed in a bag and placed behind the right liver lobe. (14) A hepatic-jejunal anastomosis was made with continuous suture on one plane using PDS No. 4-0. (15) An end-to-side pancreatojejunal anastomosis was performed, with posterior plane of pancreatic serosa to seromuscular layer of jejunal loop, duct-to-mucosa anastomosis with single monofilament sutures No. 5-0 and anterior plane with continuous suture, using monofilament No. 3-0, placing a 5 Fr. stent. (16) An end-to-side duodenojejunal anastomosis was performed on two planes, inner plane with absorbable suture No. 3-0, outer plane with monofilament No. 3-0. (17) A cholecystecomy was performed. (18) The surgical piece was removed extending the umbilical portal incision to 3cm. (19) Two lateral Penrose type drains were placed, which were removed through lateral portal sites.
The surgery time was 600min, blood loss was 300cc, transfusion of blood products was not required.
Postoperative managementBefore the patient was extubated, she was sent to the recovery room for 2h. She was then taken to the ward where she was strictly monitored for the first 24h, measuring central venous pressure, vital signs, strict fluid balance and loss through catheters and drains, maintaining urinary flow rate above 1cc/kg/min. Thromboprophlyaxis was established with anti-embolism stockings and administration of low molecular weight heparin was commenced after 24h. The urinary catheter and nasogastric tube were removed on the second day. The patient started to walk 48h postoperatively, on the third day she passed gases and on the fourth day started an oral liquid diet, which progressed 24h later. A sample was taken from the drains to determine pancreatic enzyme levels, on the third and sixth days, which reported negative for fistula. The patient was discharged on the tenth postoperative day.
The definitive histopathological report was of a moderately differentiated adenocarcinoma of the ampulla of Vater of intestinal phenotype, 3cm×2.5cm×2cm, infiltration to the pancreas and duodenal muscle layer, tumour-free surgical margins, lymphovascular permeation present, 7 lymph nodes with no metastasis PT3-N0-M0.
Ten weeks postoperatively the patient was asymptomatic with a good quality of life.
DiscussionWhipple's procedure is frequently performed in referral centres throughout the world and is one of the most complex abdominal operations. In clinics that specialise in biliopancreatic surgery, such as our institution, a protocol has been established for diagnostic approach and preoperative preparation, which has enabled appropriate surgical planning.
The laparoscopic approach for pancreatoduodenectomy is a highly complex surgical procedure, which requires experience in open biliopancreatic surgery and advanced laparoscopy skills.11 Its benefits include smaller incisions and a shorter recovery time. This technical method has shown no disadvantages compared to open surgery.
The biliopancreatic surgery clinic was started in the Hospital General de México in June 1993, after its chief surgeon had been trained in the field in Paris (France). The purpose of the clinic is to manage the principal diseases of the pancreas (acute pancreatitis, exocrine and endocrine neoplasms), in a protocolised and multidisciplinary fashion (endoscopy, interventionist radiology, endocrinology, intensive therapy, anaesthesiology, pathology). Since then the treatment outcomes of these patients have improved. More than 230 traditional and pylorus-preserving Whipple procedures have been performed, achieving the quality standards set out in the international literature for this type of surgery, with operative morbidity and mortality rates of 32% and 6.1% respectively (these data were presented at the III National Congress of the Mexican Hepato Pancreato Biliary Association, in December 2015).
With the advent of laparoscopic surgery, training protocols were started for distal pancreatectomy with splenectomy, and preservation of the spleen. The first procedure was successfully performed in 2008. Since then, the laparoscopic approach is the first choice for procedures including distal pancreatectomy or enucleation of some neuroendocrine tumours. Laparoscopic surgery to treat pancreatic pseudocysts was also developed in this period. Based on the wide experience over 20 years in pancreatic surgery, the training process for performing the Whipple procedure laparoscopically was started in 2013. This involved a first stage to include the assessment phase of the resection and the resection phase only, to then subsequently convert to open surgery for the reconstruction phase in the traditional manner. Once this goal had been achieved, and with the help of the bariatric surgery team, the process was started to perform the reconstruction phase laparoscopically. The result of this was the first successful, completely laparoscopic, pylorus-preserving Whipple procedure performed in our institution as we describe in this paper. It is appropriate to mention that many technical details of the procedure were discussed and reviewed during the procedure with surgeons experienced in both open and laparoscopic surgery. These details included creating a hepatojejunal anastomosis before a pancreatico-jejunal anastomosis and leaving the Kocher manoeuvre almost to the end.
Some adjustments with regard to the open technique had to be made to enable appropriate exposure in the laparoscopic route. Most noteworthy was leaving the Kocher manoeuvre until we had mobilised the first jejunal loop and disinserted the ligament of Treitz, as described by Asbun and Stauffer.12 Subsequently, the jejunal loop to be used to construct the biliary and pancreatic anastomosis was taken up through the site where the fixed loop was disinserted, changing the chronological order that anastomoses are first made, the hepaticojejunal anastomosis followed by the pancreatojejunal anastomosis and finally the duodenojejunal anastomosis, without altering the anatomical order (Fig. 5). Finally, the cholecystectomy was performed at the end of the procedure.
In a recent study by Tee et al.,10 analysing the results of the main published series on laparoscopic pancreatoduodenectomy, we found a report of 0% mortality in one procedure, reported by Kendrick et al. In the series by Dulucq et al. and de Palanivelu et al., the main postoperative complication was delayed gastric emptying (9%), while pancreatic fistula was the second most frequent complication (4–6%). Two series report pancreatic fistula as the commonest complication (17–27%), and delayed gastric emptying as the second (9–14%). On reviewing the 4 studies, the morbidity and mortality rates reported were 31–54% and 0–4.5%, respectively.
Various studies comparing the open and laparoscopic approaches have demonstrated the safety of the laparoscopic approach. Asbun and Stauffer12 prospectively compared 53 laparoscopic procedures with 215 open procedures and reported a significant difference in favour of the laparoscopic route, with a reduction in transoperative blood loss (195 vs. 1032cc, p<0.001), in the requirement for blood transfusion (0.64 vs. 4.7U, p<0.001), and in duration of hospital stay (8 vs. 12.4 days, p<0.001). In our case, a surgery time above that of the open approach was recorded, but with less blood loss, early start of oral diet (4th day), and shorter hospital stay (10 days), compared with the open procedure. The data reported are within international parameters.
From an oncological perspective, an R0 resection was achieved, and the number of lymph nodes were within the internationally reported range.
ConclusionFrom our point of view, this type of procedure should be performed in centres with experience in open pancreatic surgery, with training in advanced laparoscopic surgery and following a strict protocol.
The real advantages of this approach for the Whipple procedure are principally the reduced transoperative blood loss and shorter hospital stay. The long-term outcomes of this type of surgery as treatment for pancreatic and bile duct tumours remain unknown.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
To Dr. Irma Tarcila Alcocer Maldonado, for the illustrations used in the manuscript.
Please cite this article as: Chapa-Azuela O, Roldán-García JA, Díaz-Martínez J, Etchegaray-Dondé A. Pancreatoduodenectomía totalmente laparoscópica. Primer caso reportado en México. Cir Cir. 2017;85:344–349.