Diabetes mellitus 2 has become a global problem. It is estimated that 15–25% of patients could develop a chronic ulcer in their life, and nearly 33% of direct care costs of the diabetes mellitus 2 is spent on treating these ulcers. Mesenchymal stem cells have emerged as a promising cell source for the treatment of these ulcers.
Clinical caseThe case is presented of a 67 year-old male with a history of diabetes mellitus, acute myocardial infarction, and chronic ulcer involving right foot and part of his leg. He was treated with mesenchymal stem cell management, resulting in skin graft integration and full coverage of the lesion.
ConclusionThe implementation of mesenchymal stem cell techniques for treatment of chronic ulcer is feasible. The impact on the population would lead to a significant improvement in their quality of life and reduce healthcare spending.
La diabetes mellitus tipo 2 se ha convertido en un problema a nivel mundial. Se estima que del 15 al 25% de los pacientes desarrollarán una úlcera crónica a lo largo de su vida, y cerca del 33% de los costos directos de atención a diabetes mellitus tipo 2 se gasta en la atención de estas úlceras. Las células troncales mesenquimales han surgido como una fuente celular prometedora para el tratamiento de este tipo de úlceras.
Caso clínicoMasculino de 67 años con antecedentes de diabetes mellitus tipo 2, infarto agudo de miocardio y úlcera crónica en miembro inferior derecho que involucra la cara interna del pie y parte de la pierna derecha; se le trató con células troncales mesenquimales, resultando una adecuada integración del injerto de piel y la cobertura total de la lesión.
ConclusiónLa implementación de células troncales mesenquimales para el tratamiento de la úlcera crónica es factible. El impacto en la población sería importante, al mejorar la calidad de vida de los pacientes y disminuir los costos de atención.
Diabetes mellitus type 2 has become a global problem. It is estimated that in the year 2030 a 70% concentration of patients with this disease will live in developing countries, and Mexico will Rank sixth or seventh in the international league.1 If we take into consideration current trends and behaviour patterns of diabetes mellitus type 2 patients, the long-term complications of this disease, which include nephropathy, retinopathy, peripheral vascular disease and neuropathy pose a real challenge to the medical profession due to their complexity.2,3 It is estimated that 15–25% of diabetes mellitus type 2 patients will develop a chronic ulcer during their lifetime, and this population is also 10–20 times more likely to undergo non-traumatic amputation of a limb, compared with the population which does not present with this pathology.4–9 The social impact of this type of lesion must be considered, since the average duration of an ulcer is between 12 and 13 months; nearly 70% of patients who have had an ulcer will present with at least one other ulcer during their lifetime, with a consequential estimated loss of 2 million working days from inability to work.10 In 2010 alone it was estimated that Mexico spent 778,427,475 US dollars on DM2 medical attention11–17 (US$343,226,541 on direct costs and US$435,200,934 on indirect costs),18 and nearly 33% of direct care costs of diabetes mellitus type 211–17 was spent on treating chronic ulcers.19
One of the problems we encounter on treating this type of lesion is the low probability of success, since under the best conditions only 50% of all cases remit, and for the other 50% of patients the ulcers are one of the main reasons for amputation, with an estimated one-year survival for 70% of patients and five years for 20%.20 Regarding the organic characteristics leading to the ulcers, including insufficient tissue perfusion from microvasculature damage,2,3 treatment based on cells or their products (cell therapy) has recently been proposed to improve microvasculature repair mechanisms and stimulate re-epithelisation.21 These new alternatives propose a treatment based on mesenchymal stem cells, on the premise that these cells produce modulators which affect both the inflammatory response and growth factors for tissue regeneration.11,22
Mesenchymal stem cells are formed during embryonic life in the mesoderm, giving rise to connective tissue, bone, muscle and cartilage. Once the embryo has formed stem cells reserves remain almost throughout the whole body and they may be identified, as may their function, through the use of specific cell markers. These cells have been identified in bone marrow, the umbilical cord, fatty tissue and placenta tissue. Clinically they may be used in ulcers of diverse aetiology, with the exception of neoplastic ulcers.
The aim of this paper is to share our recorded experience with autologous mesenchymal stem cells as treatment for a patient with a chronic ulcer secondary to diabetes mellitus type 2.11–17
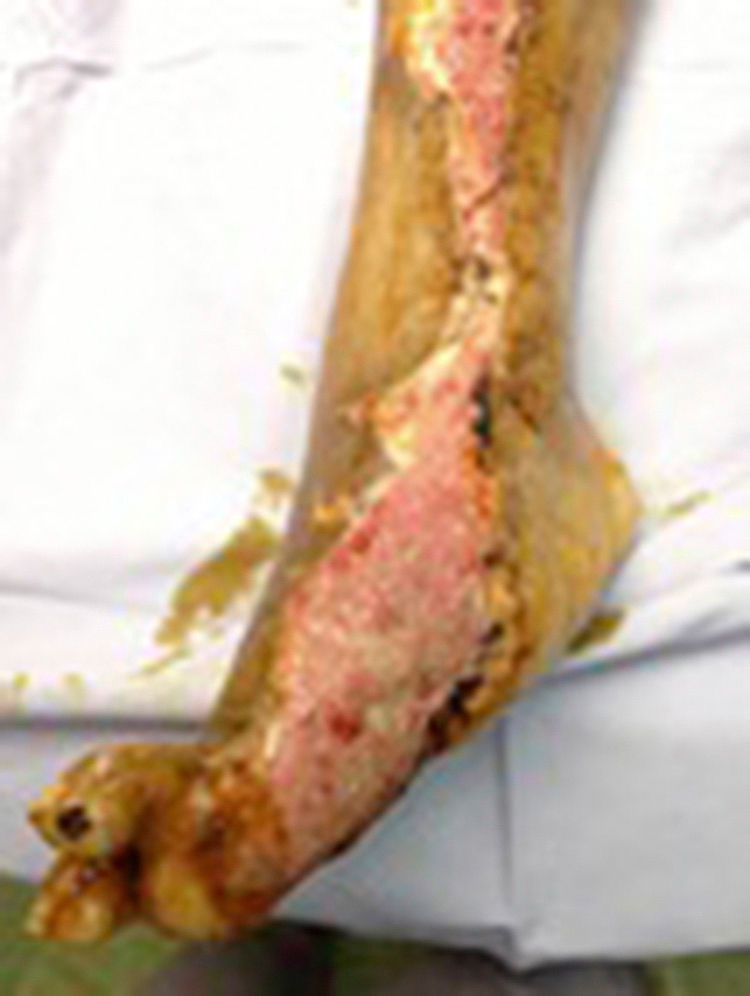
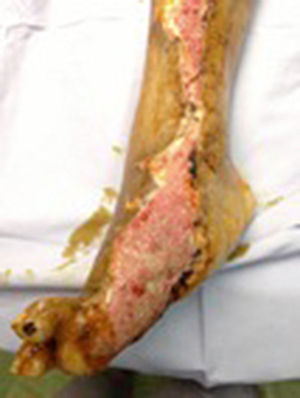
Clinical caseA 67 year old man with a 15 year history of diabetes mellitus type 2 was assessed by the Orthopaedic Unit in the Hospital General de Puebla for a chronic ulcer (of one-year duration) in the right pelvic limb, which had spread from the inner side of the foot on the sole, involving the first and second toes, up the inner side of the leg to its middle third. Culture testing of the lesion revealed Staphylococcus sp. and Escherichia coli. Given the conditions of the ulcer and the fact that this was a multi-treated patient, therapeutic amputation or the use of mesenchymal stem cells with autologous skin graft was indicated. Subsequent to describing the procedures, the risks and probabilities of both improvements from both procedures, the patient chose mesenchymal stem cells and autologous skin graft and gave his written consent to the same.
The first step consisted in keeping the patient hospitalised whilst the infection was controlled using third generation cephalosporins; an initial debridement was performed, in addition to the amputation of the first and second toes. Four further surgical interventions were performed (with 15 day spacing between each one). During the first surgical intervention scarification of the injury was made and puncture of the spinae for the obtainment of 60ml of bone marrow; citrate phosphate dextrose was used to prevent coagulation of the specimen and for cell maintenance; the specimen was then submitted for processing in the Laboratorio de Medicina Regenerativa in coordination with the Laboratorio de Morfología de la Escuela Superior de Medicina del Instituto Politécnico Nacional to obtain and cultivate the mesenchymal stem cells for 30 days.
Mesenchymal stem cell isolationThe mesenchymal stem cells were obtained by bone marrow aspiration of the ileum using a 60ml syringe with citrate phosphate dextrose as the anti-coagulant. The aspired material was diluted in a sterile medium of 1:2 parts with a solution of sterile PBS1× and centrifuged at a density gradient of NycoPrepTM 1.077g/ml, for 30min at 1500rpm. Mononuclear cells were separated from the interface and were washed on 2 occasions with PBS1× solution, centrifuged for 10min at 2000rpm and seeded into 25cm (CORNING) Petri dishes with 5ml of poietics human mesenchymal stem cells medium, at 37°C and 5% of CO2; this was supplemented with 1× antibiotic–antimicotic. After 3 days it was washed with the 1× citrate phosphate dextrose solution to remove non adherent cells and a fresh medium was added.
Once a cellular confluence of 70–80% had been obtained, the adherent cells were detached using an EDTA trypsin solution (0.025%, GIBCO) and incubated for 10min at 37°C. The cells were washed with PBS and seeded into a 2×103cell/ml solution in 100mm×20mm (CORNING) Petri dishes now with 10ml of DMEM/F-12 and an addition of 10% SFB and antibiotics. These cells were reseeded up to three times for assessment of membrane markers by flow cytometry; their plasticity/cellular differentiation to osteoblasts and adipocytes was also assessed.
Characterisation of the mesenchymal stem cells was performed by assessing the surface markers (anti-CD73, anti-CD90 anti-CD105, anti-CD14, anti-HLA-DR, anti-CD13 and anti-CD45) by flow cytometry. The results obtained showed that new cells expressed high levels of CD73, CD90, CD105 and CD13. However, they did not express haematopoietic markers such as CD45, CD14 and HLA-DR.
Cellular plasticity was assessed whilst cultivating the cells in the presence of specific differentiating media including: StemPro Adipocyte Differentiation Basal Medium and StemPro Ostecyte/Chondrocyte Differentiation Basal Medium (GIBCO) for 15 days. The results obtained indicated that our cells are able to differentiate themselves from both adipocytes and osteoblasts.
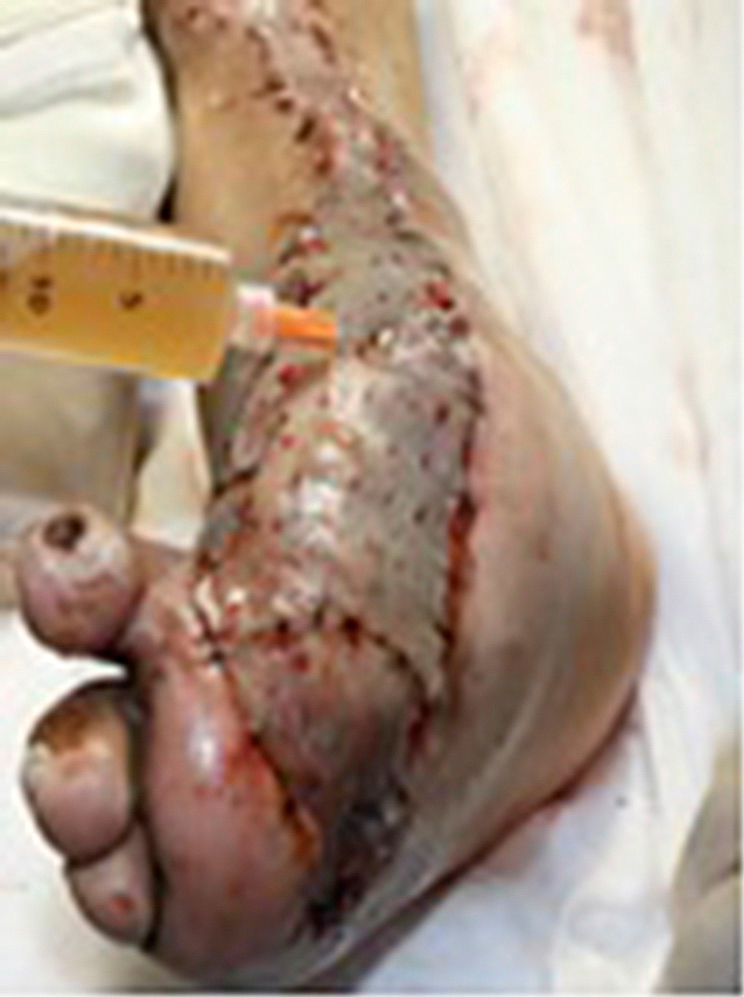
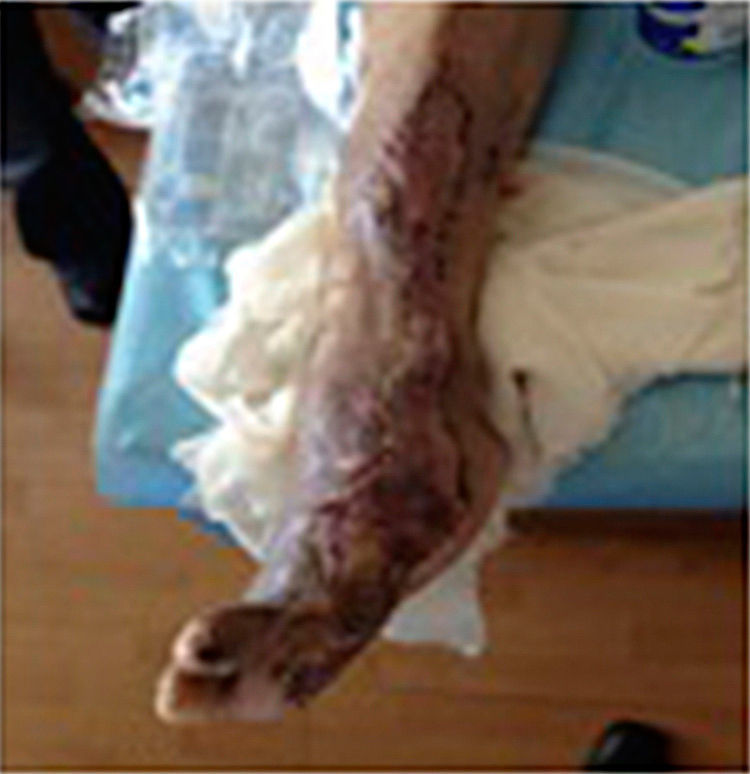
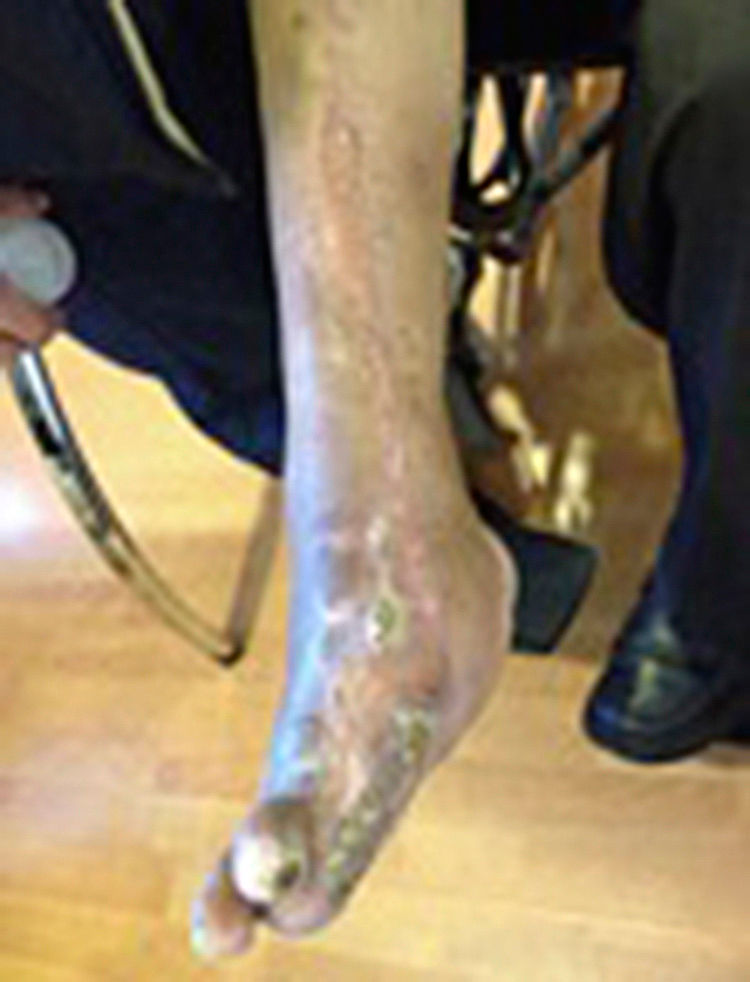
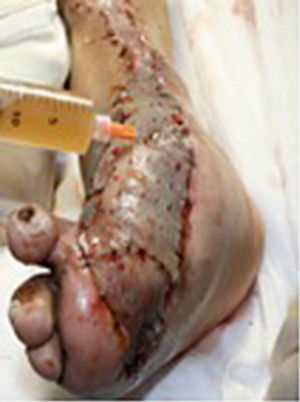
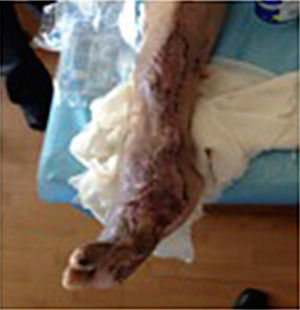
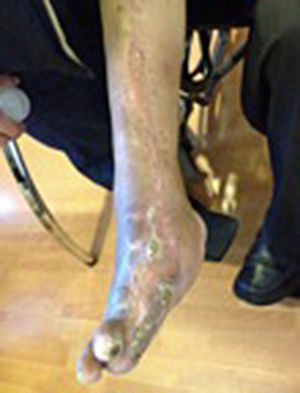
During the second and third surgical intervention surgical cleaning of the site was carried out, and during the fourth intervention the autologous mesenchymal stem cells were placed on a clean site suspended in platelet-rich plasma, with a distribution of 1 million cells per cm2, after which an autologous cutaneous graft of 15cm×10cm (mesh) taken from the ipsilateral thigh was inserted. The graft was covered with vaseline-covered gauzes and 500mg ciprofloxacin was administered for 2 weeks. The patient was assessed on a weekly basis, with the total integration of the graft being documented 2 weeks later; 6 months later the patient was able to put weight on the limb, and after a year he continued to be complication free. The patient's evolution is shown in Figs. 1–4.
It is of note that the preparation of autologous mesenchymal stem cells from bone marrow was carried out in the Laboratorio de Medicina Regenerativa y Cáncer in coordination with the Laboratorio de Morfología, both from the Escuela Superior de Medicina del Instituto Politécnico Nacional, complying with criteria established by the Sociedad Internacional de Terapia Celular (ISCT), which includes verification of identity by flow cytometry expressing main (positive) markers CD105, CD73, CD90 and not expressing mainly (negative) CD45, CD34, HLA-DR12 ones, in addition to trypan blue verification of cellular viability at 95% and confirming the presence of a bacteria-free solution through a culture, and with the verification of the cellular morphology under a simple microscope. Moreover, as a control measure, an aliquot of the autologous mesenchymal stem cells was taken from the wound, which were later recovered, sown and multiplied again in the laboratorio de Medicina Regenerativa y Cáncer of the Instituto Politécnico Nacional, with confirmation of their biological characteristics (Fig. 5).
DiscussionDiabetes mellitus type 211–17 and its complications is a major financial drain on global health systems, and Mexico is no exception. It will rank high among countries in 2030 and economic pressure for care resulting from this disease is increasing disproportionately.8,19 Chronic ulcers are one of the main complications, and are one of the main causes of amputation due to complex management and treatment.6–10,18–20 During the last 3 decades the rise in cellular therapies has enabled other therapeutic options to become available, thus changing this scenario to such an extent that they are now considered to be better cost-effective options,13,14 and are now included in international ulcer management guidelines such as those from Sociedad de Atención de Heridas,15–17 and those of several hospital protocols, such as the Mount Sinai Hospital in New York.
Mesenchymal stem cells have been successfully used with this type of lesion due to their ability to regulate inflammatory processes and regenerate tissue through factors such as interleukin-10 and interleukin-4, in addition stimulating new vessel growth by vascular growth factor, growth factor derived from platelets, recruiting of keratinocytes, markings for the migration of native stem cells and finally remodelling of the wound through regulation of keratinocytes and the presence of growth factor β and metaloproteinases,23 key elements for tissue regeneration.
The transdisciplinary approach enabled this patient to preserve his limb. Correct disease management by the medical and surgical team from the orthopaedic Unit of the Hospital General de Puebla to control infection and clean the site, together with the obtainment of autologous mesenchymal stem cells from the Laboratorio de Medicina Regenerativa y Cáncer, in coordination with the Laboratorio de Morfología, both from the Escuela Superior de Medicina of the Instituto Politécnico Nacional with the highest quality characteristics, enabled acceptance of the graft by the patient and the ulcer wound bed to be covered. Under other conditions the limb would have been amputated. The existence of strong cooperation between the transdisciplinary team was key in this regard, since if one person does not appropriately carry out his or her role the success of treatment may be compromised.
This type of transdisciplinary approach has enabled patients to be treated in a non conventional therapeutic manner, offering them an alternative option to amputation.
ConclusionThe use of a cell-based therapy – and in this particular case mesenchymal stem cells – is a global reality with benefits for both patients and health care systems. The use of these techniques is feasible in our area and its impact on the population affected by this type of disease would be high, with subsequent improvement in quality of life and lower health care expenditure.
Conflict of interestsThe authors have no conflict of interests to declare.
To Dr. Roberto J. Robles Ramirez for his support in the bacterial cultures, to Q.F.B. Leonardo Fermín Acevedo Olvera for his support in the cellular cultures and to C.P.R. Dr. Gerardo Rayón Nieva for his invaluable collaboration.
Please cite this article as: Benítez-Arvízu G, Palma-Lara Í, Vazquez-Campos R, Sesma-Villalpando RA, Parra-Barrera A, Gutiérrez-Iglesias G. Células troncales mesenquimales autólogas e injerto cutáneo autólogo para tratamiento de una úlcera crónica secundaria a diabetes mellitus tipo 2. Cir Cir. 2015;83:532–536.