Complex regional pain syndrome is characterised by spontaneous or induced pain disproportionate in relation to the initial event and is accompanied by a variety of regional and motor disturbances, leading to a variety of clinical presentations. It is often associated with surgery and minor trauma.
PathophysiologyThree mechanisms are postulated: changes secondary to post traumatic inflammation, peripheral vasomotor dysfunction and structural and functional changes of the central nervous system as a result of maladaptation.
DiagnosisIt is made based on the criteria of Budapest. The patient must have one symptom and sign of each criterion at diagnosis: continuing pain, disproportionate to any inciting event. A sensory, vasomotor, oedema and motor/trophic change sign and symptoms that are not explained by another diagnosis or cause.
TreatmentMultimodal treatment is suggested. There is no gold standard. In early stage NSAIDs or steroids can be used. Drugs used for neuropathic pain treatment have been suggested, but there is not enough evidence for any of these. There is low evidence that bisphosphonates, calcitonin, ketamine and mirror therapy are effective compared to placebo. Interventional treatment should be stepped from epidural block, neurostimulation, intrathecal pump to experimental therapies in case of intractable pain.
DiscussionAlthough complex regional pain syndrome has been a recognised entity for over 100 years, no clear evidence exists for first-line treatments; however, new technologies that are applicable in complex regional pain syndrome treatment have been developed.
El síndrome doloroso regional complejo se caracteriza por dolor espontáneo o inducido, desproporcionado con relación al evento inicial y que se acompaña de una gran variedad de alteraciones autonómicas y motoras, dando lugar a una gran variedad de presentaciones clínicas. Con frecuencia se asocia a cirugías y a traumatismos menores.
FisiopatologíaSe postulan 3 mecanismos: cambios por inflamación postraumática, disfunción vasomotora periférica y cambios funcionales y estructurales del sistema nervioso central secundarios a una mala adaptación.
DiagnósticoSe realiza tomando como base los criterios de Budapest. El paciente debe presentar un síntoma de cada criterio al momento del diagnóstico: dolor continuo, desproporcionado en relación con cualquier evento desencadenante. Un signo y un síntoma: sensorial, vasomotor, edema y cambio motor/trófico. Por último, estos no se explican por otro diagnóstico o causa.
TratamientoSe sugiere que sea multimodal. No existe un estándar de oro. En fase temprana se pueden utilizar AINE o esteroides. Se han indicado fármacos utilizados para tratamiento de dolor neuropático, pero ninguno de estos posee suficiente evidencia. Hay baja evidencia de la efectividad de que los bifosfonatos, la calcitonina, la ketamina y la terapia en espejo sean efectivos comparados con placebo. El tratamiento intervencionista debe ser escalonado de bloqueo peridural, neuroestimulación, bomba intratecal hasta las terapias experimentales en caso de dolor refractario a tratamiento.
DiscusiónA pesar de que el síndrome doloroso regional complejo es una entidad reconocida desde hace más de 100 años, todavía no existe evidencia clara en las primeras elecciones terapéuticas, aunque hay nuevas tecnologías aplicables en su tratamiento.
A pain syndrome secondary to changes in the sympathetic nervous system was first described by Claude Bernard in 1851.1 Thirteen years later, in 1864, Mitchell, Morehouse and Keen2,3 wrote a detailed publication on severe pain accompanied by oedema, changes in skin colour and temperature, hyperalgesia and joint stiffness in patients with gunshot wounds, which they named causalgia. At the beginning of the 20th century Sudeck reported rapid progression of pain symptoms accompanied by bone atrophy presenting similarly to causalgia, after a soft tissue injury and other types of trauma.4
Through the years this syndrome has been known as causalgia, Sudeck's atrophy or dystrophy, algodystrophy, algoneurodystrophy, shoulder-hand syndrome reflex neurovascular dystrophy, causalgia and reflex sympathetic dystrophy.5,6 Evans first used the term “sympathetic dystrophy” to cover these disorders in 1946. In 2001, Schott proposed the term “complex regional pain syndrome” (CRPS), which was accepted by the International Association for the Study of Pain (IASP). This is currently the most accepted medical term for this clinical picture.7
CRPS is defined by the IASP as a disorder characterised by spontaneous and induced pain, disproportionate in relation to the initial event and accompanied by a great variety of autonomic and motor disturbances, resulting in a large diversity of clinical presentations.8
Annual incidence varies according to the population and the study, ranging from 5.47 to 26.2 cases/100,000 people. The lowest reported incidence is 5.46/100,000 inhabitants in a study performed in the USA between 1989 and 1999.8 Whereas in the Netherlands the incidence of CRPS was 20/100,000 inhabitants.9
A gender ratio of 3:1, 4:1, is reported, the condition is always more common in females. CRPS has been classified into 2 types, depending on whether or not there has been prior nerve damage or injury. In terms of subtype, it has been reported that around 85% of cases are type 1 where it has not been possible to identify a nerve lesion, and only 13% are type 2. Due to the clear lack of knowledge with regard to its pathophysiology, the question has been raised as to whether CRPS comprises many subtypes, all with different aetiologies and pathogeneses but with a similar presentation, included within two pain syndromes.10 It could affect patients who have undergone surgery for carpal tunnel syndrome or Dupuytren's contracture.
Being female, especially postmenopausal, a dislocated ankle or intra-articular fracture, immobilisation and severe pain present in the early phase of the trauma have been posited as potential risk factors for developing CRPS type 1.11
PathophysiologyThree pathophysiological mechanisms for CRPS have been postulated. The first attaches great importance to changes due to post-traumatic inflammation, the second proposes peripheral vasomotor dysfunction and the third theory suggests that the central nervous system presents functional and structural changes secondary to poor adjustment to chronic pain.12
Posttraumatic inflammationMost inflammatory changes in this syndrome are mediated by calcitonin gene-related peptide (CGRP) and substance P, because in patients with CRPS the concentrations of these peptides are higher than in healthy individuals. The increase in CGRP might be responsible for increased neuronal hyperexcitability. Neurogenic inflammation, the mechanism by which the inactivation of neuropeptides in impeded and receptor availability is increased, might present due to postneuronal signalling and extravasation of proteins. In vitro, substance P stimulates keratinocytes to express proinflammatory cytokines, therefore facilitating neuropeptide signalling in skin contributes directly to inflammation, extravasation, limb oedema and subsequent increased expression of inflammatory cytokines.
Increased tumour necrosis factor alpha and inteleukin-6 concentrations in skin biopsies of the affected limb have not been associated with the clinical signs or duration of the disorder. However they have been associated with the extension of the mechanical hyperalgesia phenomenon, i.e., with exaggerated response to pain caused by pressure.
Mechanical hyperalgesia, present in CRPS, is a hallmark in central sensitisation. Inflammatory cytokines probably act beyond the affected limb, i.e., in the spinal cord. Here they might sensitise secondary nociceptive neurons or through glial-neuronal interactions. Two studies have found increased concentrations of interleukin-1β and interleukin-6 in the cerebrospinal fluid of patients with chronic CRPS (7–8 years duration).13
Similarly, an autoimmune mechanism has been posited, since 35% of patients present surface-binding autoantibodies against sympathetic and mesenteric plexus neurons. However, the clinical relevance of this has not been determined.
Vasomotor dysfunctionChanges in temperature in the affected limb in patients with this syndrome indicate changes in the vasoconstrictor neurons over time. Three different temperature patterns have been identified.
A warm temperature pattern, caused by an apparent failure in body cooling due to the impossibility of activating the vasoconstricting neurons. An intermediate temperature pattern, the characteristic pattern in patients with CRPS of 28 months’ duration, where the limb might be warmer or cooler depending on the degree of sympathetic activation. And finally the cold pattern, where the temperature and perfusion of the affected limb is lower than that of the contralateral limb. The latter is probably due to the unilateral inhibition of cutaneous sympathetic vasoconstrictor neurons, caused by functional changes at the level of the spinal/cerebral medulla, triggered by the initial trauma. Norepinephrine levels might not be associated with the phenomenon of vasoconstriction because no differences have been found in the concentration of this catecholamine between the affected side and the healthy side. However, sympathetic-afferent coupling, i.e., norepinephrine released by the sympathetic nerve fibres that activates or sensitises the afferent neurones, might cause the phenomenon of sympathetic maintenance pain.
In patients with CRPS there are also changes in endothelial function associated with a reduction in nitric oxide release, which causes sustained vasoconstriction.
Central sensitisationThe sensitisation process distorts or suppresses non-nociceptive sensations. The loss of inhibitory flow generated by normal skin sensations in the affected limb causes excitability of the talamocortical nociceptive pathway which causes perpetuation of pain between the reduction of the inhibitory influence and the facilitation of the excitatory systems that project to the rostroventral medulla.
Dystonia, the most prevalent symptom of CRPS, and present from the early stages of the condition, is characterised by postures of flexion and persistent inversion. Because dystonia does not respond to intravenous ketamine infusion it is thought that it is secondary to changes in neuroplasticity resulting from maladaptative neuroplasticity. The nature of this mechanism is not well understood, but the GABAB receptor might play an important role since administration of its agonist, baclofen, improves dystonia in patients with CRPS.
In functional imaging techniques, the reorganisation of the somatotopic map inside the primary somatosensory cortex (S1) can be seen contralateral to the affected limb. S1 representation of the affected hand is less than that of the healthy hand. The degree of these changes relates to spontaneous pain and mechanical hyperalgesia. S1 cortical reorganisation can be seen in patients who start treatment and their pain improves. A reduction of the inhibitory mechanisms can also be observed and increased excitability of the contralateral primary motor cortex in patients with CRPS.14
Very probably changes in cortical function contribute towards the sensory loss frequently observed in patients with CRPS. However, the cortical reorganisation demonstrated in patients with chronic CRPS is not enough make this pathological process the therapeutic target. The fact is that the pathophysiological mechanism of this syndrome has not been completely explained.15 The same applies to the large range of clinical manifestations and causes as evident as a CRPS picture presenting after a surgical procedure or trauma and as unique and rare as those presenting after a laser discectomy.16
DiagnosisThere is no gold standard for diagnosis; therefore it is difficult to reach a definitive diagnosis of CRPS. Diagnosis is based principally on clinical symptoms. Application of the Budapest criteria is currently the most accepted method for diagnosing CRPS. The Budapest consensus group introduced the following criteria for identifying patients with CRPS and excluding other neuropathic conditions.17
The IASP committee recently approved and coded the taxonomy of the Budapest criteria as “the new IASP criteria”. These are described in Table 1.
Budapest diagnostic criteria for CRPS.
| (1) Continuing pain, disproportionate to any inciting event |
| (2) A patient must report at least one symptom in 3 of the following categories |
| Sensory: hyperaesthesia and/or allodynia |
| Vasomotor: temperature asymmetry and/or skin colour changes and/or skin colour asymmetry |
| Sudomotor/oedema: oedema and/or sweating changes and/or sweating asymmetry |
| Motor/trophic: decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin) |
| (3) At least 1 sign must be present at the time of evaluation in 2 or more of the following categories |
| Sensory: hyperalgesia (to pinprick) and/or allodynia (to light touch and/or deep somatic pressure and/or joint movement) |
| Vasomotor: temperature asymmetry and/or skin colour changes and/or asymmetry |
| Sudomotor/oedema: oedema and/or sweating changes and/or sweating asymmetry |
| Motor/trophic: decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin) |
| (4) No other diagnosis can explain the signs and symptoms |
Within the Budapest criteria for diagnosing CRPS, it should be born in mind that signs or symptoms should be present at the time of diagnosis. The patient must report one of the symptoms in each of the 4 categories in order for a clinical diagnosis to be made.18 The diagnostic criteria are shown in Table 1.
The intention of the Budapest criteria is to maximise specificity (minimise false positives) at the expense of sensitivity. They have high specificity but low sensitivity.
The absence of a gold standard makes it difficult to validate the diagnostic criteria.
Laboratory studies can be used in the different stages of CRPS to support a diagnosis. Thermometer and Doppler measurement of vasomotor tone are used to evaluate vasomotor changes. Bone densitometry and skin biopsy are useful for the measurement of atrophy and reduction of nerve density. Rheumatological studies evaluate inflammatory arthritis. Electrodiagnostic tests serve to assess the peripheral nervous system, which can help in diagnosing CRPS type 2. Plain x-rays can reveal advanced osteoporosis or fracture in the limb with CRPS symptoms. Magnetic resonance evaluates soft tissue injuries and bone oedema.
Differential diagnoses include: infectious arthritis, inflammatory arthropathy, peripheral arteriopathy and deep vein thrombosis. In the chronic phase (with sequelae): Dupuytren's disease, sclerodermia and plantar fascitis. Coxitis and osteonecrosis should be ruled out in disorders of the hip. If there is bone demineralisation it is recommended that hip fractures, osteoporosis and benign and malignant bone tumours should be ruled out.19
TreatmentTreatment of CRPS is multimodal. It includes conservative, pharmacological and interventionist management. There is debate as to the treatment of choice since there is no evidence to support any as first choice.
Non-pharmacological treatmentA conference was held in Malibu, California in 1997 towards reaching a consensus on treatment for the functional restoration of CRPS. Physical and occupational therapy were suggested as initial treatment, leaving drugs, psychological therapy and intervention as second line.18
The objective of physical and occupational therapy is to minimise oedema, desensitise the pain in the limb and normalise sensitivity, encourage normal positions, reduce muscle mass loss and increase the function of the limb.
Bandages or compression sleeves are used to reduce oedema along with manual techniques for oedema mobilisation. Water therapy has also been shown to reduce oedema and improve weight bearing ability.19
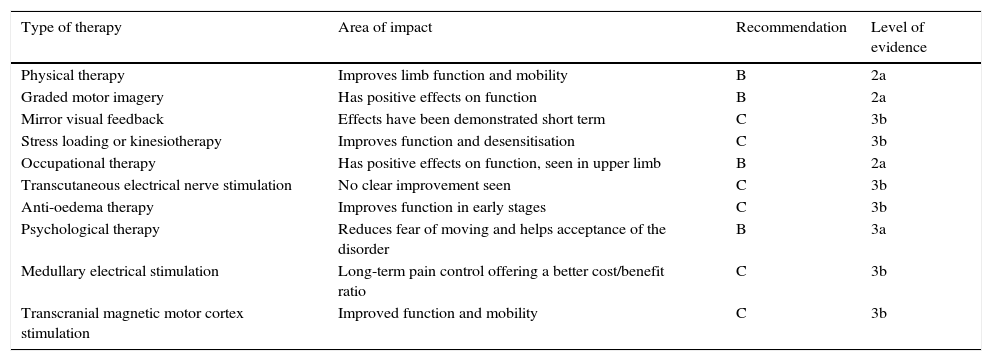
Conservative measures. Rehabilitation and other therapiesThe objective of early rehabilitation is to prevent pain-associated disability and limited mobility. One of the most used therapies is active and passive kinesiotherapy which comprises gentle movement and increased sensory stimuli such as rubbing with objects of different textures or temperature. Physiotherapy and rehabilitation are effective, and there is strong evidence that they promote improvement in function and pain for CRPS patients. There is strong evidence for graded motor imagery therapy in patients with complex regional pain syndrome after wrist fracture. There is moderate evidence for mirror therapy on its own.
Occupational therapy has positive effects on functional limitation and levels of activity. Electrotherapy or transcutaneous electrical nerve stimulation has also been recommended for improving pain, but with a lower level of evidence compared to kinesiotherapy and occupational therapy. There is insufficient evidence with the use of transcutaneous electrical nerve stimulation.19
Tables 2 and 3 show the different conservative and pharmacological treatments with their level of recommendation and their level of evidence.18,20 The levels of recommendation are described as follows: A (systematic revisions and meta-analysis of clinical trials and individual clinical trials with strict confidence intervals), B (systematic revisions of cohort and case–control studies, individual cohort studies and case-control studies, and clinical trials of poorer quality), C (poor quality case series and cohort and case–control studies), D (expert opinion). Level of evidence 1 refers to meta-analyses and systematic revisions; Level 2 refers to one or more randomised studies, controlled with a good sample; 3 refers to retrospective or pilot studies and 4 to clinical cases, anecdotal cases and clinical experience.
Conservative therapies in CRPS.
| Type of therapy | Area of impact | Recommendation | Level of evidence |
|---|---|---|---|
| Physical therapy | Improves limb function and mobility | B | 2a |
| Graded motor imagery | Has positive effects on function | B | 2a |
| Mirror visual feedback | Effects have been demonstrated short term | C | 3b |
| Stress loading or kinesiotherapy | Improves function and desensitisation | C | 3b |
| Occupational therapy | Has positive effects on function, seen in upper limb | B | 2a |
| Transcutaneous electrical nerve stimulation | No clear improvement seen | C | 3b |
| Anti-oedema therapy | Improves function in early stages | C | 3b |
| Psychological therapy | Reduces fear of moving and helps acceptance of the disorder | B | 3a |
| Medullary electrical stimulation | Long-term pain control offering a better cost/benefit ratio | C | 3b |
| Transcranial magnetic motor cortex stimulation | Improved function and mobility | C | 3b |
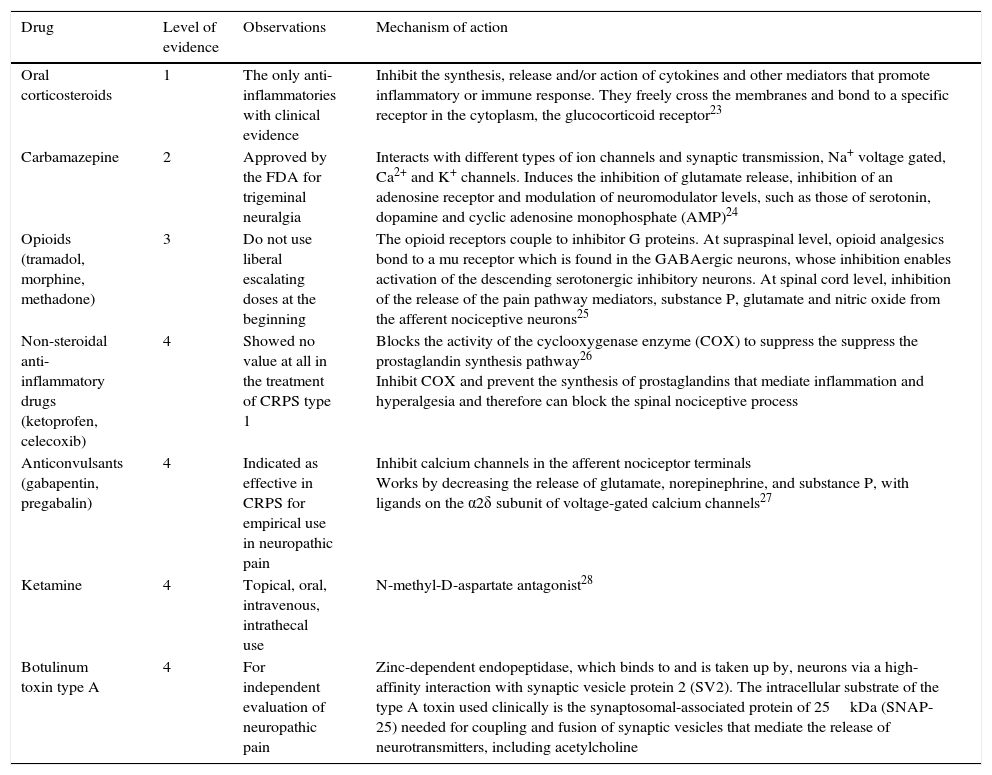
Drug therapy in CRPS.
| Drug | Level of evidence | Observations | Mechanism of action |
|---|---|---|---|
| Oral corticosteroids | 1 | The only anti-inflammatories with clinical evidence | Inhibit the synthesis, release and/or action of cytokines and other mediators that promote inflammatory or immune response. They freely cross the membranes and bond to a specific receptor in the cytoplasm, the glucocorticoid receptor23 |
| Carbamazepine | 2 | Approved by the FDA for trigeminal neuralgia | Interacts with different types of ion channels and synaptic transmission, Na+ voltage gated, Ca2+ and K+ channels. Induces the inhibition of glutamate release, inhibition of an adenosine receptor and modulation of neuromodulator levels, such as those of serotonin, dopamine and cyclic adenosine monophosphate (AMP)24 |
| Opioids (tramadol, morphine, methadone) | 3 | Do not use liberal escalating doses at the beginning | The opioid receptors couple to inhibitor G proteins. At supraspinal level, opioid analgesics bond to a mu receptor which is found in the GABAergic neurons, whose inhibition enables activation of the descending serotonergic inhibitory neurons. At spinal cord level, inhibition of the release of the pain pathway mediators, substance P, glutamate and nitric oxide from the afferent nociceptive neurons25 |
| Non-steroidal anti-inflammatory drugs (ketoprofen, celecoxib) | 4 | Showed no value at all in the treatment of CRPS type 1 | Blocks the activity of the cyclooxygenase enzyme (COX) to suppress the suppress the prostaglandin synthesis pathway26 Inhibit COX and prevent the synthesis of prostaglandins that mediate inflammation and hyperalgesia and therefore can block the spinal nociceptive process |
| Anticonvulsants (gabapentin, pregabalin) | 4 | Indicated as effective in CRPS for empirical use in neuropathic pain | Inhibit calcium channels in the afferent nociceptor terminals Works by decreasing the release of glutamate, norepinephrine, and substance P, with ligands on the α2δ subunit of voltage-gated calcium channels27 |
| Ketamine | 4 | Topical, oral, intravenous, intrathecal use | N-methyl-D-aspartate antagonist28 |
| Botulinum toxin type A | 4 | For independent evaluation of neuropathic pain | Zinc-dependent endopeptidase, which binds to and is taken up by, neurons via a high-affinity interaction with synaptic vesicle protein 2 (SV2). The intracellular substrate of the type A toxin used clinically is the synaptosomal-associated protein of 25kDa (SNAP-25) needed for coupling and fusion of synaptic vesicles that mediate the release of neurotransmitters, including acetylcholine |
The lack of a gold standard diagnostic test or a specific mechanically-based diagnostic system has greatly hindered well-designed trials, and there is little evidence to guide the treatment of these patients.
The presence of neuropathic bone pain has been posited. Therefore drugs have been studied, used specifically for CRPS, which interfere with bone metabolism, particularly in inhibiting osteoclasts that are in charge of bone resorption. These medications include calcitonin and biphosponates.21 Other drugs such as corticoids have been used and more recently intravenous immunoglobin.
Drug treatments that have been most studied in other related neuralgias include tricyclics, gabapentin and pregabalin, carbamazepine, opioids, clonidine, nifedipine, α-adrenergic antagonists, 5% lidocaine patch, and topical capsaicin.18
Non-steroidal anti-inflammatory drugs, corticosteroids, cyclooxigenase-2 inhibitors and free radical scavengers are used for pain with the intention of treating inflammatory disorders in CRPS.
Non-steroidal anti-inflammatory drugs have demonstrated no value at all in the treatment of CRPS1. Selective cyclooxygenase-2 inhibitors (celecoxib, for example), have not been tested in CRPS, although anecdotally they have been reported as being of some use (level of evidence 4).22
Oral corticosteroids are the only anti-inflammatory drugs for which there is direct clinical evidence in CRPS (level of evidence 1).18,19
Neuromodulators such as gabapentin and pregabalin are suggested to be effective for CRPS (level of evidence 4). These are used empirically for many neuropathic pain syndromes.
Carbamazepine holds a traditional place in the treatment of trigeminal neuralgia. A randomised controlled study of patients with CRPS found that 600mg/day of carbamazepine, taken over more than 8 days, resulted in considerable pain reduction compared to the placebo (evidence level 2).
There is growing consensus that although opioids are a second or third line of treatment as a reasonable treatment option, they should not be used initially and the dose should not be liberally escalated. Methadone has theoretical advantages for neuropathic pain due to its putative N-methyl-d-aspartate antagonism, and the practical advantage of low cost. Tramadol can be useful with its concomitant blocking serotonin–norepinephrine reuptake.
Ketamine, N-methyl-d-aspartate receptor antagonist, has been used topically, orally and intravenously (and recently, intrathecally, not supported by evidence) in various doses to treat neuropathic pain, particularly CRPS.18
Table 3 summarises the different drugs used to manage CRPS according to the latest guidelines from Harden et al.18
Botulinum toxin type A used to weaken specific muscles in movement and spasticity disorders by blocking the release of acetylcholine in the cholinergic synapses, also inhibits the cholinergic neurotransmitters (glutamate for example) and neuropeptides (P substance and calcitonin gene-related peptide [CGRP], related to the primary afferent nerve terminals, providing the bases for independent assessment of neuropathic pain. However in CRPS there is insufficient evidence that botulinic toxin is effective for treating dystonia.29
Interventionist treatmentIn the 4th edition of the CRPS guidelines by Harden et al.18 the evidence is revised for the following interventions: sympathetic nervous system blocks, peridural block with local anaesthetic and neuromodulation.
When the condition affects the thoracic limbs, the block is made at the level of the stellate ganglion and the sympathetic lumbar chain for the pelvic limbs.
There are really no indications with established criteria for performing these types of procedures.
This systematic review revealed the shortage of published trials supporting the use of local anaesthesia in sympathetic block as a gold standard for CRPS. The 2 randomised studies that meet the inclusion criteria had small sample sizes, and consequently no conclusions could be drawn about the effectiveness of this procedure. Randomised controlled studies are needed to evaluate the usefulness of sympathetic block with local anaesthesia in the treatment of CRPS.30,31
Brachial and spinal plexus blocksPlacing a catheter at the level of the brachial plexus for a period of 3 weeks and the continuous administration of local anaesthetic, clonidine, opioids and other agents have reported better pain control, with evidence level 4.18
Sympathetic block with chemical and thermal neurolytic agentsRadiofrequency ablation has been revisited recently in case series, obtaining a level of evidence 3.32 According to Cochrane, the practice of chemical and surgical sympathectomy for neuropathic pain and CRPS is based on very few high quality trials. Sympathectomy should be used with caution in clinical practice, in carefully selected patients and probably only after other treatment options have failed, particularly at cervical level.33
Dorsal root ganglion stimulationIt is postulated that the pathophysiological changes in the dorsal root ganglion might be a contributory factor towards the development of CRPS, and therefore its stimulation might be of some therapeutic benefit. This therapeutic alternative involves placing stimulation electrodes at the level of the intervertebral foramen, i.e., adjacent to the dorsal root ganglion.17 A case series33 has been published recently in which 8 patients diagnosed with CRPS according to the Budapest criteria, underwent dorsal root ganglion neuromodulation after a trial period with a reduction of pain equal or greater than 50%. Although the results were variable, all the subjects presented an improvement in their pain and some had remission of sympathetic maintenance symptoms.34,35
NeurostimulationPosterior cord stimulation is based on the gate control theory that non-noxious stimuli such as paresthesia travel through long fast-conducting fibres, inhibiting impulses from small diameter fibres (like the pain fibres). Spinal cord stimulation can improve non-nociceptive pain increasing GABAergic activity in the spinal column, thus reducing peripheral sensory excitation and increasing peripheral vasodilatation.
Kemler et al. published a randomised study on patients under conservative management, patients with neurostimulation alone, with follow-up at 6 months, and reported a greater reduction in pain and subjective impact on improved quality of life. However they did not demonstrate an improvement in the patients’ functional status.36
There is limited evidence for long-term response to implantation systems. In a study with a 5 year follow-up after implantation, 40% of patients still had 30% pain improvement after 11 years.37
According to the latest revision by Cochrane38 on interventions to improve capacity in patients with CRPS, interventionist options can be classified according to Table 4.
Minimally invasive procedures for the treatment of CRPS.
| Moderate level of evidence | Low level of evidence | Very low level of evidence | |
|---|---|---|---|
| Ineffective strategies | Intravenous block with guanethidine | Sympathetic block with local anaesthesia | Intravenous mannitol, gabapentin, relaxation therapy, manual lymph drainage |
| Effective strategies | Ketamine, biphosphonates and calcitonin vs. placebo Mirror therapy | Corticosteroids vs. placebo Clonidine (peridural and intravenous) Sympathetic block with botulinum toxin Spinal cord stimulation and physical therapy vs. physical therapy |
There are clinical cases which report a good response to autologous stem cell and platelet-rich plasma implantation, with follow-up at 30 days.39
Although the prognosis of patients with CRPS is very difficult to predict, timely diagnosis and treatment increase the likelihood of a successful outcome. Mild cases respond to physiotherapy, while moderate cases can require adjuvant analgesics such as gabapentinoids and/or antidepressants.2
DiscussionDespite the fact that CRPS has been an acknowledged entity for more than 100 years, there is still no clear evidence for the primary therapeutic choices, whether non-pharmacological, pharmacological or interventionist, although we should mention that there are currently promising new applicable technologies. Medicine is constantly evolving, as is this disorder. For example, the diagnostic tests which were previously considered the most reliable for diagnosing the sympathetic component, such as sympathetic inhibition with phentolamine, are now no longer recommended due to the adverse events they involve.17,40 Moreover, in acknowledging the dynamics of how medicine is developing scientifically we must ensure that future research in this and other fields pinpoint evidence to enable better decision-making.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Hernández-Porras BC, Plancarte-Sánchez R, Alarcón-Barrios S, Sámano-García M. Síndrome doloroso regional complejo: revisión. Cir Cir. 2017;85:366–374.