Large vessel sarcomas are rare tumours. Leiomyosarcoma of the inferior vena cava is the most common. About 300 cases have been reported in the literature. They tend to be large, and not develop metastasis. The prognosis of these tumours is poor.
Clinical caseAn 81-year-old woman who complained of pain in the right flank, with no other symptoms. Abdominal computed tomography showed a large retroperitoneal mass, which affected the inferior vena cava, with signs of thrombosis inside. It also encompassed the right renal vein and the right kidney. Excision of the tumour was performed in block, performing an autologous saphenous vein bypass between left the renal vein and proximal segment of inferior vena cava.
DiscussionLeiomyosarcomas of the inferior vena cava are classified according to their relationship with adjacent structures. The clinical signs and symptoms are generally non-specific. Diagnosis is made using computed tomography or magnetic resonance imaging, and biopsy of the retroperitoneal mass. Surgery is the only treatment capable of providing prolonged survival. The surgical management is determined by: the level of involvement, the extension, and the presence or absence of collateral veins. The role of adjuvant therapy is controversial.
ConclusionsInferior vena cava leiomyosarcomas remain a challenge for surgeons. At present, radical resection with negative margins, offers the highest survival rate. The best results are obtained with a multidisciplinary approach by experienced teams in the management of these tumours.
Los sarcomas de grandes vasos son tumores raros; el leiomiosarcoma de cava inferior es el más frecuente de ellos, del que existen unos 300 casos descritos. Tienden a presentar gran tamaño sin causar metástasis. El pronóstico de estos tumores es malo.
Caso clínicoMujer de 81 años que consulta por dolor en fosa renal derecha, sin otra sintomatología. La tomografía computada abdominal muestra una gran masa retroperitoneal, que compromete a la vena cava inferior, con signos de trombosis en su interior; igualmente, engloba a la vena renal derecha y al riñón derecho. Se realizó exéresis de la tumoración en bloque, con un bypass autógeno, con safena entre vena renal izquierda y segmento proximal de vena cava inferior.
DiscusiónLos leiomiosarcomas de vena cava inferior se clasifican en función de su relación con las estructuras vecinas. La clínica suele ser inespecífica. El diagnóstico se realiza por tomografía computada o resonancia magnética nuclear y biopsia de la masa retroperitoneal. La cirugía es el único tratamiento capaz de proporcionar supervivencias prolongadas. El manejo quirúrgico se determina por: el nivel de afectación, la extensión y la presencia o ausencia de venas colaterales. El papel del tratamiento adyuvante es controvertido.
ConclusionesLos leiomiosarcomas de vena cava inferior continúan siendo un desafío para los cirujanos. En la actualidad, la resección radical con márgenes negativos ofrece la mayor tasa de supervivencia. Los mejores resultados se obtienen con un abordaje multidisciplinario por parte de equipos experimentados en el manejo de estos tumores.
Large vessel sarcomas are rare. The most common origin is the inferior vena cava, although some authors consider those of the pulmonary artery and the aorta more common. The neoplasms that occur in these areas are angiosarcomas, intimal sarcomas and leiomyosarcomas. The latter is the most common.1,2 Leiomyosarcoma represents 0.5% of all sarcomas that affect adults and comprises less than one in 100,000 of all malignant tumours. Leiomyosarcoma is a malignant tumour of mesenchymal origin that develops from the smooth muscle cells of the tunica media.1 This tumour tends to be large in size without causing metastasis and to grow throughout the lumen of the vessel.2 Since it was described by Pearl in 1871, around 300 cases have been reported located in the inferior vena cava; they are more common in women in the 6th decade of life.2 Due to their insidious symptoms, they are most often an incidental finding in the form of a retroperitoneal mass. Currently possibility of a cure can only be offered by surgery.3 The prognosis of these patients is poor.
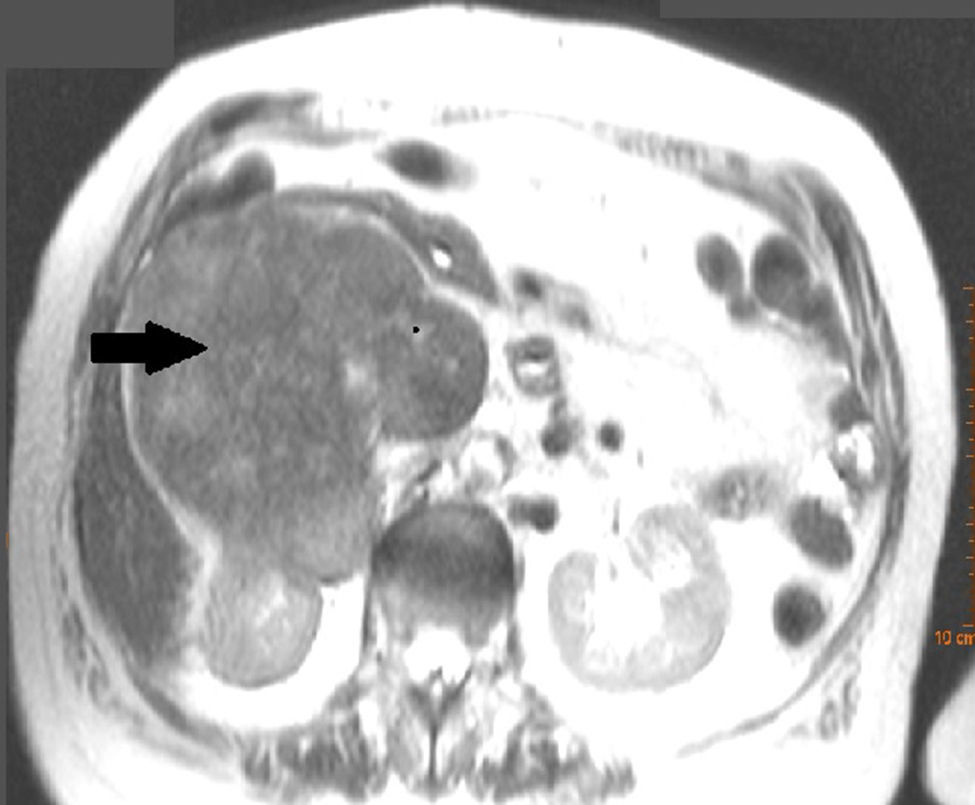
Clinical caseWe present the case of an 81-year-old woman, with no medical or surgical history of interest, who consulted with pain in the right renal fossa, not associated with other symptoms. The initial laboratory report showed normal parameters of renal function and elevated LDH. Abdominal ultrasound showed a large solid mass, of approximately 13cm in sagittal diameter, which was infiltrating the medial wall of the right kidney. Occupation of the lumen could be seen and dilation of the right renal vein and inferior vena cava, compatible with tumour thrombosis. Abdominal computed tomography (CT) (Fig. 1) showed a large heterogeneous retroperitoneal mass, with areas of necrosis with diameters of 14cm×11cm×10cm, which was compromising the inferior vena cava, which was increased in size with signs of tumour thrombosis in one segment extending from the level of the renal veins to their infrahepatic portion. In the same way, it was invading and causing thrombosis of the right renal vein, extending through the renal hilum in intimate contact with the medial border of the right kidney. There was collateral circulation, with increased calibre of the azygos and hemiazygos veins and varices in the uterine veins, and no significant adenopathies. A magnetic resonance scan was performed, showing a mass situated in the right pararenal space, measuring approximately 12.3cm×10.7cm×14cm, with heterogeneous signal intensity and signs of infiltration into the right renal sinus and the inferior vena cava, which was markedly dilated with a signal intensity similar to that of the tumour mass. Fine needle aspiration biopsy reported a pleomorphic leiomyosarcoma.
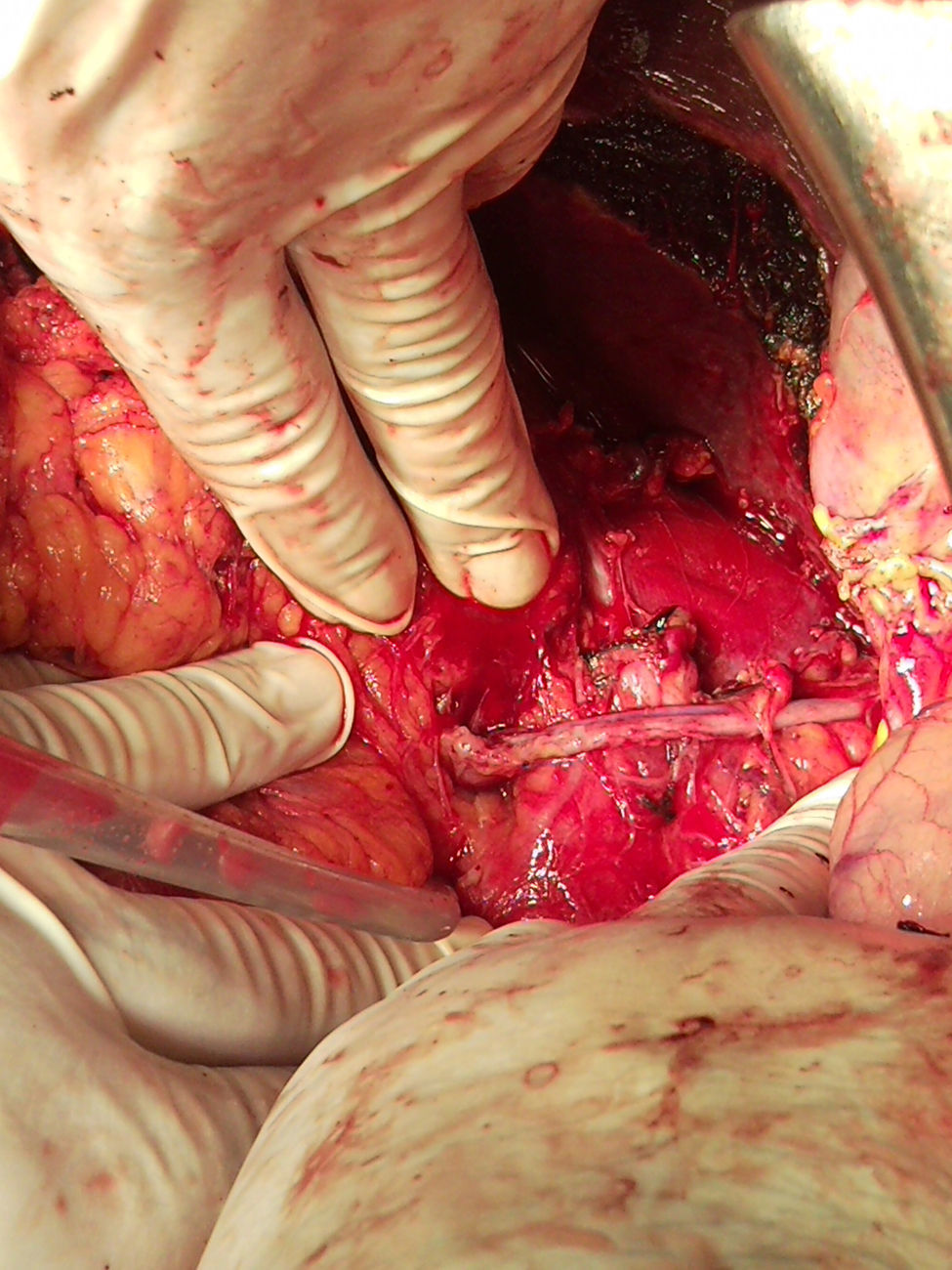
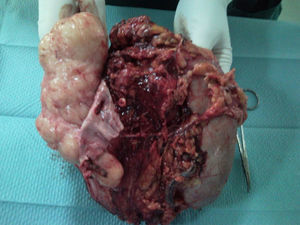
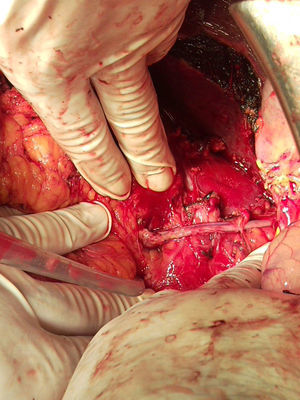
Surgery was scheduled to remove the lesion. A midline laparotomy was performed and a large retroperitoneal mass was observed encompassing the right kidney and part of the inferior vena cava. After vascular control at the lower and upper area of the mass, the inferior vena cava was opened, it was totally occupied by the tumour which completely covered the entrance to the left renal vein and extended to the area close to the retrohepatic portion. An en bloc resection was performed of the inferior vena cava situated above the iliac bifurcation, to the area immediately below the inferior border of the liver, including the right kidney and the entire retroperitoneal mass (Fig. 2). The resection included a small liver resection in segments IV and V. A bypass was made with the internal saphenous vein, from the left renal vein to the inferior vena cava proximal to the tumour, to ensure venous drainage of the left kidney (Fig. 3). The patient had odynophagia due to candidiasis as a postoperative complication. She was discharged with no sequelae.
Pathological anatomy reported an irregular tumour of 1.320g in weight infiltrated by the tumour. The resection borders were not affected by the tumour, although they were close at some areas (1mm at the upper area). There were no areas of necrosis or haemorrhage. Immunohistochemistry was positive for smooth muscle desmin and actin and negative for HMB-45 antibody, and therefore the suspected diagnosis was confirmed and the possibility of an angiomyolipoma was discounted. It was not possible to assess the number of mitoses in the material submitted.
The patient was referred to Oncology, where radiotherapy was decided due to the proximity of the resection borders, 50Gy per fraction, 25 days, on the surgical bed, administered and tolerated well. After 9 months follow-up, several liver and lung lesions appeared on a control CT that had not existed before and were classified as metastases. Five months later, the patient presented symptoms of severe liver and respiratory failure, which resulted in death.
DiscussionMalignant tumours of the large vessel walls are rare. The most common of these tumours is leiomyosarcoma which principally affects the inferior vena cava. Leiomyosarcoma arises from the smooth muscle cells of the vessel, which can grow intra- and extraluminally, involving neighbouring structures.2,4,5 Leiomyosarcoma of the inferior vena cava are classified according to their relationship with adjacent structures. Level 3 describes tumours located between the right atrium and the entrance to the suprahepatic veins (16% of cases). Level 2 extends from the suprahepatic veins to the entrance to the renal veins (46% of cases) and level 1 corresponds to the infrarenal inferior vena cava (38% of cases).1,4,6 In our case, the tumour occupied levels 1 and 2.
Symptoms are non-specific and they can often be asymptomatic. In 66% of cases there is abdominal pain. Other possible symptoms include the presence of a palpable mass, oedema of the lower limbs, weight loss, Budd-Chiari syndrome, dyspnoea, nausea and vomiting, inferior cava syndrome, etc.3,4 In our patient, despite the fact that the tumour completely occupied the lumen of the inferior vena cava and was obstructing the entrance to the renal veins, there was no kidney failure or symptoms of inferior vena cava syndrome, probably because the tumour was slow growing and enabled collaterals to develop. CT and MRI provide information on the site of origin and the relationship of the tumour with the neighbouring structures. Typically, these tumours appear as heterogeneous masses with enhancement of the periphery of the tumour and can present areas of haemorrhage and necrosis.4 In general, a preoperative biopsy is necessary to confirm diagnosis. Imaging-guided core needle biopsy is the procedure of choice.5
Complete surgical resection is the only therapeutic option which can provide survival long term. Patients with unresectable tumours have a survival rate of months and palliative resections can temporarily relieve symptoms but do not offer long-term survival.4 Traditionally, the limit of resectability was involvement of the suprahepatic veins, although in cases where there is only involvement of the suprahepatic portion of the inferior vena cava or of only one hepatic vein, interventions with hepatectomy associated with resection of the inferior vena cava and anastomosis of the health healthy hepatic vein to a graft have been described.1 The tumour should be considered unresectable if there is involvement of the superior mesenteric vessels.5
Surgical management is determined by the level of involvement, extension and the presence or absence of collateral veins. The surgical options comprise partial resection and cavoplasty with bovine pericardial patch, complete resection with placement of a graft, and ligation of the inferior vena cava.6,7 Small tumours that affect <75% of the circumference of the inferior vena cava can be treated with partial resection of the inferior vena cava wall and cavoplasty with a bovine pericardial patch or other venoplasty patch. Tumours that affect more than 75% of the circumference of the inferior vena cava require complete resection of the segment of inferior vena cava and reconstruction using a graft.
At present, ringed polytetrafluoroethylene prostheses are the most accepted for reconstructing the inferior vena cava. Placement is recommended of a prosthesis of a calibre slightly smaller than that of the inferior vena cava to be replaced, to increase flow velocity. Some authors even recommend making an inguinal arteriovenous fistula to increase flow velocity and thereby improve the permeability of the graft.7 However, at the moment, there is no evidence that arteriovenous fistulae improve this permeability. PTFE graft infection is an uncommon but serious problem, which can occur in cases where a concomitant intestinal resection is performed. In situations of major contamination, an autologous vein graft can be used or simple ligation of the inferior vena cava.4
Tumours that totally occupy the lumen of the inferior vena cava and have developed a good network of collaterals can be treated by ligation of the inferior vena cava. This procedure cannot be used in level 3 tumours invading the inferior vena cava, but can be safely performed in tumours of levels 1 and 2.5 In level 2 tumours that affect the entrance to the renal veins, the left renal vein can be ligated, consigning venous drainage of the left kidney to the collateral veins (lumbar, gonadal and adrenal). The right renal vein can be reimplanted in the remaining vena cava or in a graft, or renal autotransplantation performed in the right iliac fossa.1 In our case, the tumour was completely occupying the circumference of the vessel, totally obstructing the lumen of the vena cava over time. Because of this the patient had developed good venous drainage through collateral veins, and therefore the decision was made not to reconstruct using a PTFE graft.
There is no consensus as to the need for anticoagulant or antiaggregant treatment after surgery. Some authors advocate anticoagulation with coumarin drugs for 6 months, followed by prophylactic antiaggregation,1 while others report good outcomes without anticoagulation.7
The role of neoadjuvant and adjuvant treatment is controversial. Kieffer et al.7 consider complete resection followed by adjuvant chemotherapy optimal for treating tumours with no metastasis at the time of diagnosis. However, clinical case reports and small retrospective series of patients treated with adjuvant chemotherapy after surgery have not demonstrated benefits in terms of survival or disease-free periods. The clinical guidelines of the National Comprehensive Cancer Network (NCCN)8 for retroperitoneal/intra-abdominal sarcoma, state that preoperative chemotherapy is an acceptable alternative in tumours that are not amenable to resection, but with their recommendation category 2B.4 If after chemotherapy there is regression of the tumour, surgery is recommended. The benefits of preoperative radiotherapy would be a reduction of the tumour mass in order to improve resectability. However, there are no randomised studies that have demonstrated its efficacy in improving disease-free survival.4 Currently the NCCN8 guidelines give preoperative radiotherapy a category 2B. Postoperatively, radiotherapy should be given to patients with an R0 (complete resection, with no residual tumour), with high-grade tumours, very large tumours and those with resection margins close to the tumour, as in our case. After R1 resection (incomplete resection, with histological tumour residues) the NCCN recommend postoperative radiotherapy, when it has not been given preoperatively, or a boost of 10–16Gy if radiotherapy was given preoperatively (recommendation category 2B).8
The survival rate at 2 and at 5 years of patients with resection and tumour free margins is 90% to 66.7% respectively. Survival of patients after total resection of a tumour with positive margins is 21 months and 8 months, when resection is incomplete.9 The factors influencing a greater death rate due to the disease are involvement of the upper segments of the inferior vena cava and high histological grade of the tumour. Tumours limited to level 2 present better survival than those at level 1.10 Patients with unresectable tumours have a survival of months; follow-up is performed using CT. Distant metastasis is most common in the lungs. Surgery for recurrence or metastases followed by adjuvant chemo- or radiotherapy, should be considered in patients with long disease-free periods and limited disease, and in good general condition.4 Variables associated with better outcomes and survival are: radical removal of the tumour, the presence of abdominal pain and absence of palpable mass.10,11
ConclusionsLeiomyosarcomas of the inferior vena cava still pose a challenge to surgeons. Radical resection with negative margins currently offers the best survival rate. Due to the rarity of these tumours, the best outcomes are achieved with a multidisciplinary approach by teams experienced in the management of these tumours.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: López-Ruiz JA, Tallón-Aguilar L, Marenco-de la Cuadra B, López-Pérez J, Oliva-Mompeán F, Padillo-Ruiz J. Leiomiosarcoma de vena cava inferior. Caso clínico y revisión bibliográfica. Cir Cir. 2017;85:361–365.