Mucormycosis is a rare fungal infection of the mucosa, which mainly affects immunocompromised patients. The association with emphysematous gastritis, produced by gas -forming bacteria, is rare and often fatal. The case is presented of a trauma patient with type 1 diabetes mellitus, and diabetic ketoacidosis complicated with gastric mucormycosis associated with emphysematous gastritis.
Clinical caseA male aged 32, who was involved in a car accident, and suffered head trauma, is admitted to the Intensive Care Unit, presenting with diabetic ketoacidosis and upper gastrointestinal bleeding. An endoscopy was performed, finding an erosive esophagitis Class C, ischaemia and gastric necrosis. Computed tomography scan showed emphysematous gastritis and gastric necrosis. He underwent total gastrectomy with a histopathology report of gastric mucormycosis. After the surgical procedure the patient died due to sepsis secondary to pulmonary mucormycosis
DiscussionMucormycosis is a rare fungal disease which infrequently affects the gastrointestinal tract, with the stomach being the most affected site. The mortality is high if the diagnosis is not made promptly and appropriate treatment is given.
ConclusionSuspecting its existence is necessary in patients with immunocompromised status to diagnose it and provide timely treatment to increase survival, due to its high mortality.
La mucormicosis es una infección micótica rara de la orden de los mucorales, la cual afecta en su mayoría a pacientes inmunocomprometidos. La asociación con gastritis enfisematosa es muy poco frecuente y, a menudo, es fatal producida por bacterias formadoras de gas. Presentamos el caso de un paciente politraumatizado con diabetes mellitus tipo 1 y cetoacidosis diabética que se complicó con mucormicosis gástrica asociada a gastritis enfisematosa.
Caso clínicoPaciente varón, de 32 años de edad, que sufre un accidente automovilístico, ocasionando traumatismo craneoencefálico. Ingresa a la Unidad de Cuidados Intensivos presentando cetoacidosis diabética y, posteriormente, sangrado de tubo digestivo alto. Se realiza una endoscopia, encontrándose esofagitis erosiva clase C, isquemia y necrosis gástrica. Una tomografía axial computada mostró gastritis enfisematosa y necrosis gástrica. Se sometió a gastrectomía total, reportándose en el estudio histopatológico mucormicosis gástrica. Posterior al evento quirúrgico, con desenlace fatal secundario a sepsis por mucormicosis pulmonar.
DiscusiónLa mucormicosis es una enfermedad fúngica rara, que afecta con poca frecuencia al tubo digestivo, siendo el estómago el sitio de mayor afectación. Su mortalidad es elevada si no se realiza su diagnóstico y se da tratamiento oportuno.
ConclusiónEs necesario sospechar su existencia en pacientes con estado de inmunodepresión para diagnosticarlo y dar el tratamiento oportunamente para incrementar la sobrevida, ya que cursa con elevada mortalidad.
Zygomycosis is an infection caused by fungi of the zygomycetes class, composed of mucorales and entomophthorales. Mucormycosis is an opportunistic fungal infection caused by a glomeromycota class agent of the mucorales, which mostly affects immunocompromised hosts in industrialized or developing areas1. According to Jung et al.2, the infection was first described as a pulmonary infection in 1876 by Furbringer, and the disseminated form was described in 1885 by Paltauf. Rhizopus species are the predominant human pathogens commonly found on the floor, in animal faeces and in decaying organic matter such as vegetables. Any organ system may be affected; pulmonary and rhino-orbital-cerebral infections are the most frequent3. It is an angioinvasive pathology1,4. The most frequent mechanism of transmission is the inhalation of spores, followed by the traumatic implantation of fungi elements through burns or cutaneous lesions; and the rarest direct transmission is the intravenous mechanism5. Underlying diseases cause an immunocompromised condition, particularly with neutrophil or macrophage leukocyte dysfunction considered to be the predispositioning factors6. Mononuclear and polymorphonuclear phagocytes in healthy hosts eliminate the mucorales fungi by the generation of oxidative metabolites and cationic defensins, the greatest mechanism of defence against mucormycosis. Hyperglycaemia and acidosis are known for affecting neutrophils’ chemotaxis1,5. Other pathological conditions are known to promote the infection; such is the case in the use of drugs such as deferoxamine, corticosteroids, transplanted patients, severe malnutrition or the presence of any immunocompromise. However, it has been observed in patients without the described factors7. Gastrointestinal presentation is the less frequent clinical presentation; it is particularly rare in industrialized countries. It represents between 4 and 7% of the presentations; of said presentations, only 57.7% affect the stomach, followed by involvement of the colon and the ileum. It is diagnosed ante mortem in 25% of cases, with a mortality of 85 and 95% in disseminated condition7.
Emphysematous gastritis is characterized by gas in the stomach wall due to the invasion of gas-forming microorganisms. The most commonly involved microorganisms are the streptococci Escherichia coli (E. coli), Pseudomonas aeruginosa, Clostridium perfrigens (C. perfrigens) and Staphylococcus aureus (S. aureus). The predisposing factors include the intake of corrosive substances, alcohol abuse, recent abdominal surgery, diabetes and the immunocompromised condition8. The isolation of fungi in emphysematous gastritis cases has been rare. Until now, few reports on emphysematous gastritis secondary to mucormycosis have been found, and the results are fatal9.
We present the case of a polytraumatised patient with type 1 diabetes mellitus and diabetic ketoacidosis, aggravated by gastric mucormycosis associated to emphysematous gastritis, and a review of the medical literature.
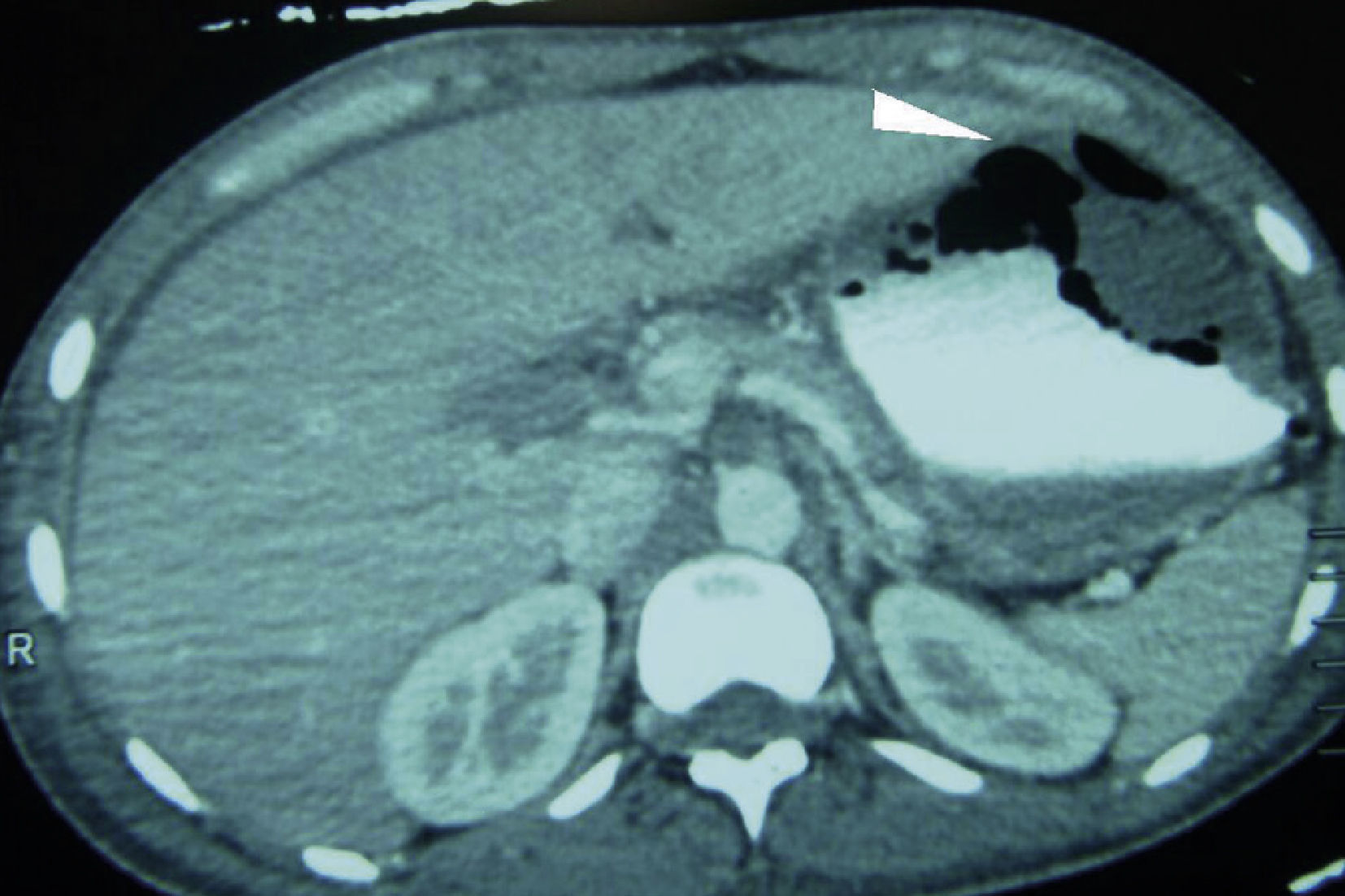
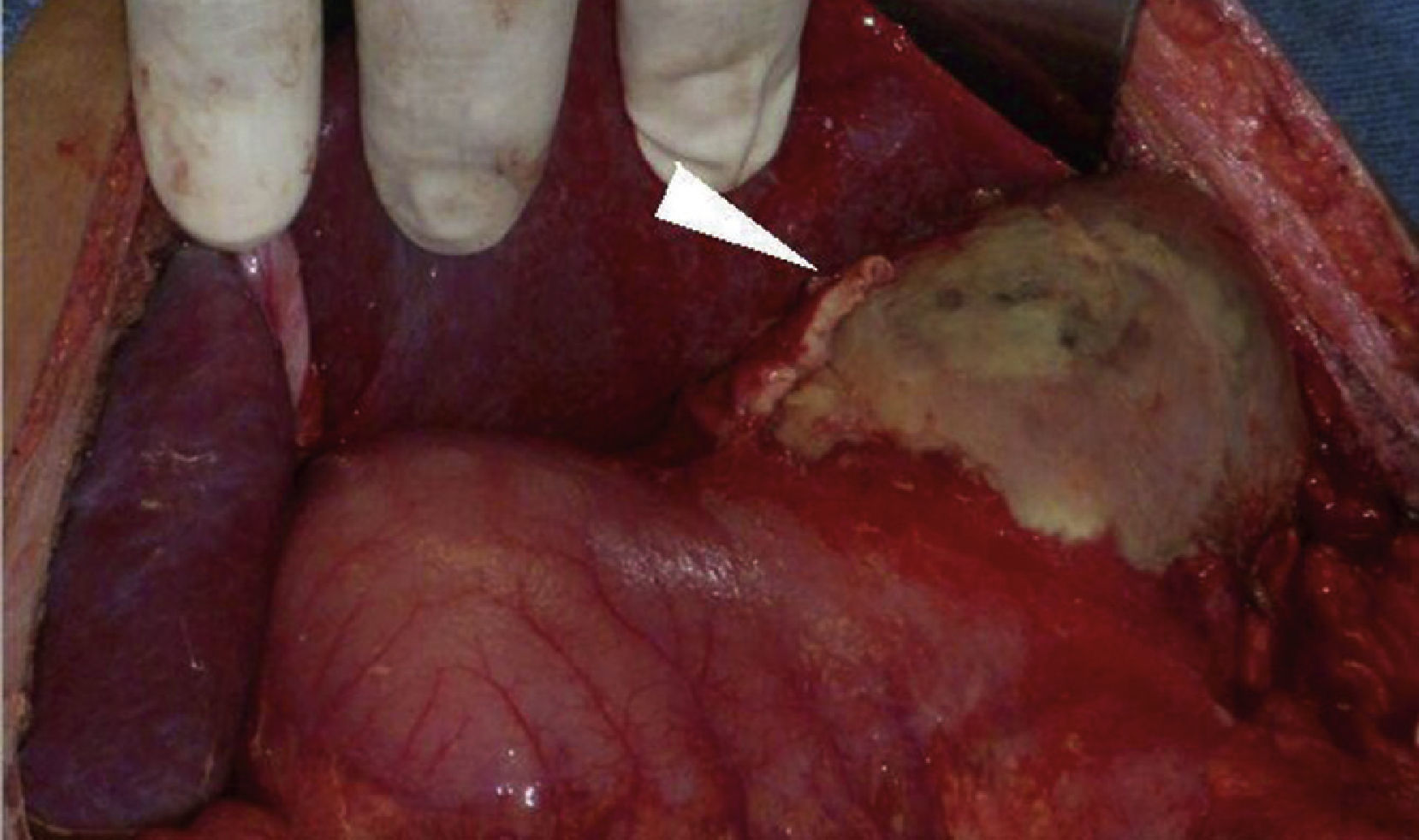
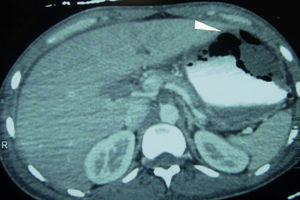
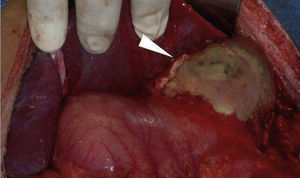
Clinical caseMale patient, aged 32 years, suffering from type 1 diabetes mellitus for seven years, managed with NPH insulin. Patient had a poly-trauma from a car accident, suffering head trauma, which required drainage of subdural haematoma and polytransfusions, as well as left tibia and fibula and right ankle fractures. He was admitted to the Intensive Care Unit with diabetic ketoacidosis. He was also bleeding in the upper digestive tract in the form of haematemesis. In the physical examination, a slight pallor of the teguments, pulmonary fields with crackling subscapularis, depressible abdomen, mild pain in the epigastrium and no peritoneal irritation were observed. Presenting leukocytosis (133/μl), neutrophilia (94%) and respiratory alkalosis (pH 7.54, PCO 2 30 mmHg, HCO3 25 mEq/l). Abdominal x-ray with radiopacity in the topography of the gastric chamber, with volume increase. The endoscopy reported oesophagus with linear lesions from middle third, covered with off-white-coloured fibrin; stomach with mucosa in the fundus and gastric body necrosis, greyish-greenish fibrin with loss of folds, antral mucosa within normal range. The abdominal tomography showed bilateral pleural effusion, gastric pneumatosis in fundus and body, perigastric oedema associated with emphysematous gastritis (Fig. 1). The patient underwent surgery. A complete gastrectomy was performed with closing of the distal oesophagus, duodenal exclusion, jejunostomy, and oesophagostomy. A pleural drainage was performed using endorpleural catheter, as foetid liquid from inflammatory reaction was found in the cavity produced by gastric necrosis in the fundus, body and greater curvature together with posterior gastritic ulcer of approximately 10×10 cm involving fundus and body of the stomach and an abscess located between the segments I and II of the liver and the superior pole of the spleen (Fig. 2). Patient was admitted to the Intensive Care Unit with aminergic and ventilation support, antibiotic therapy (cefepime, levofloxacin) and antifungal medicine (fluconazol), with APACHE II score of 30 points for mortality higher than 80%.
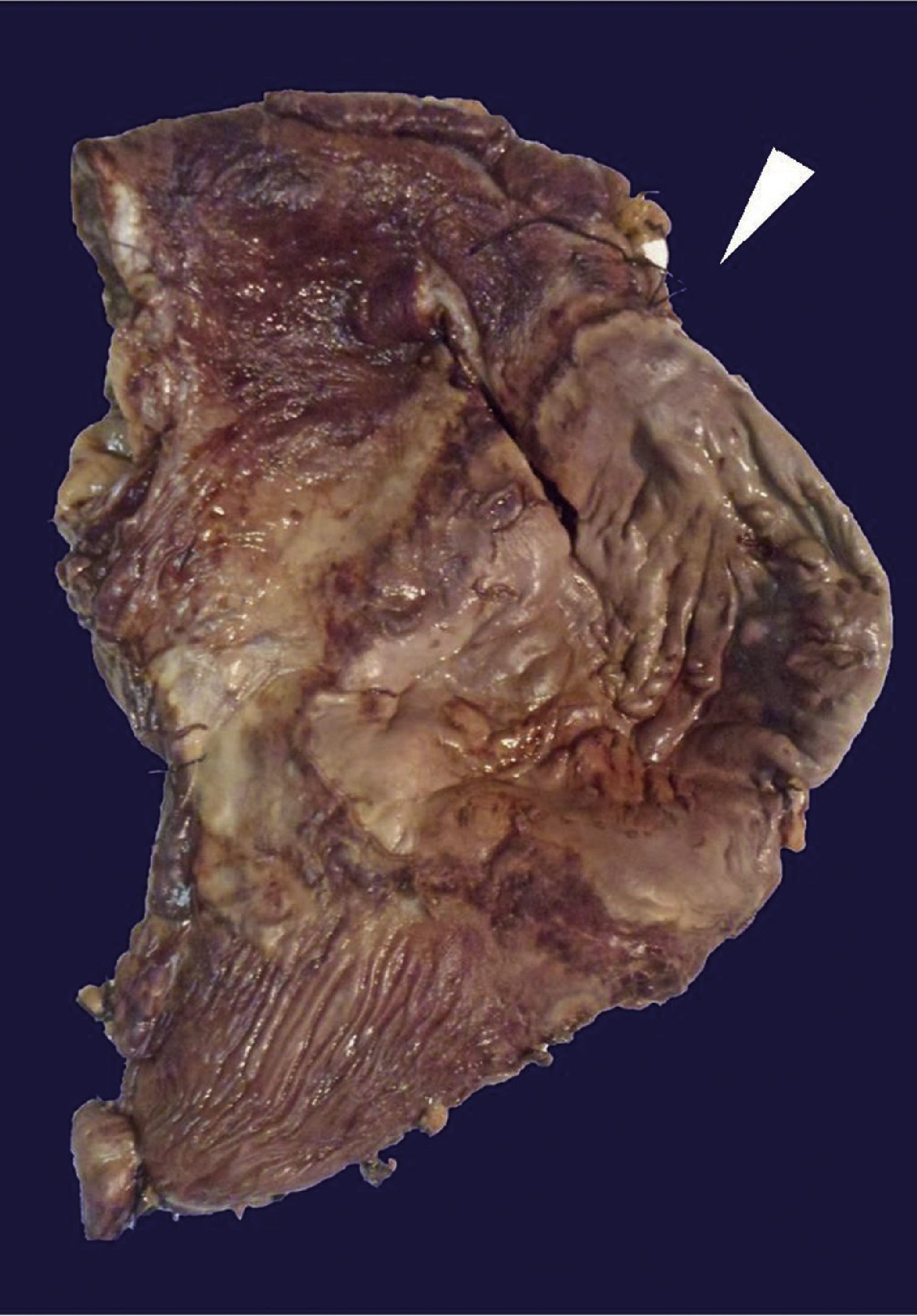
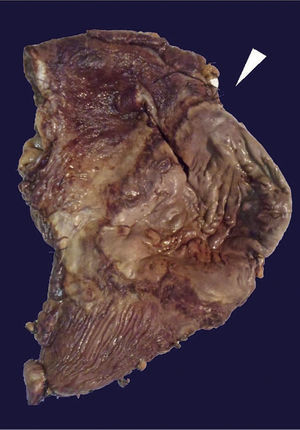
The histopathological study of the surgical piece revealed an anterior gastric ulcer of 10×9 cm at the level of the greater curvature and another ulcer in the posterior wall of 16×7 cm of a brownish-greenish colour; the rest of the gastric chamber showed a thickened wall, approximately 1 cm of thickness (Fig. 3).
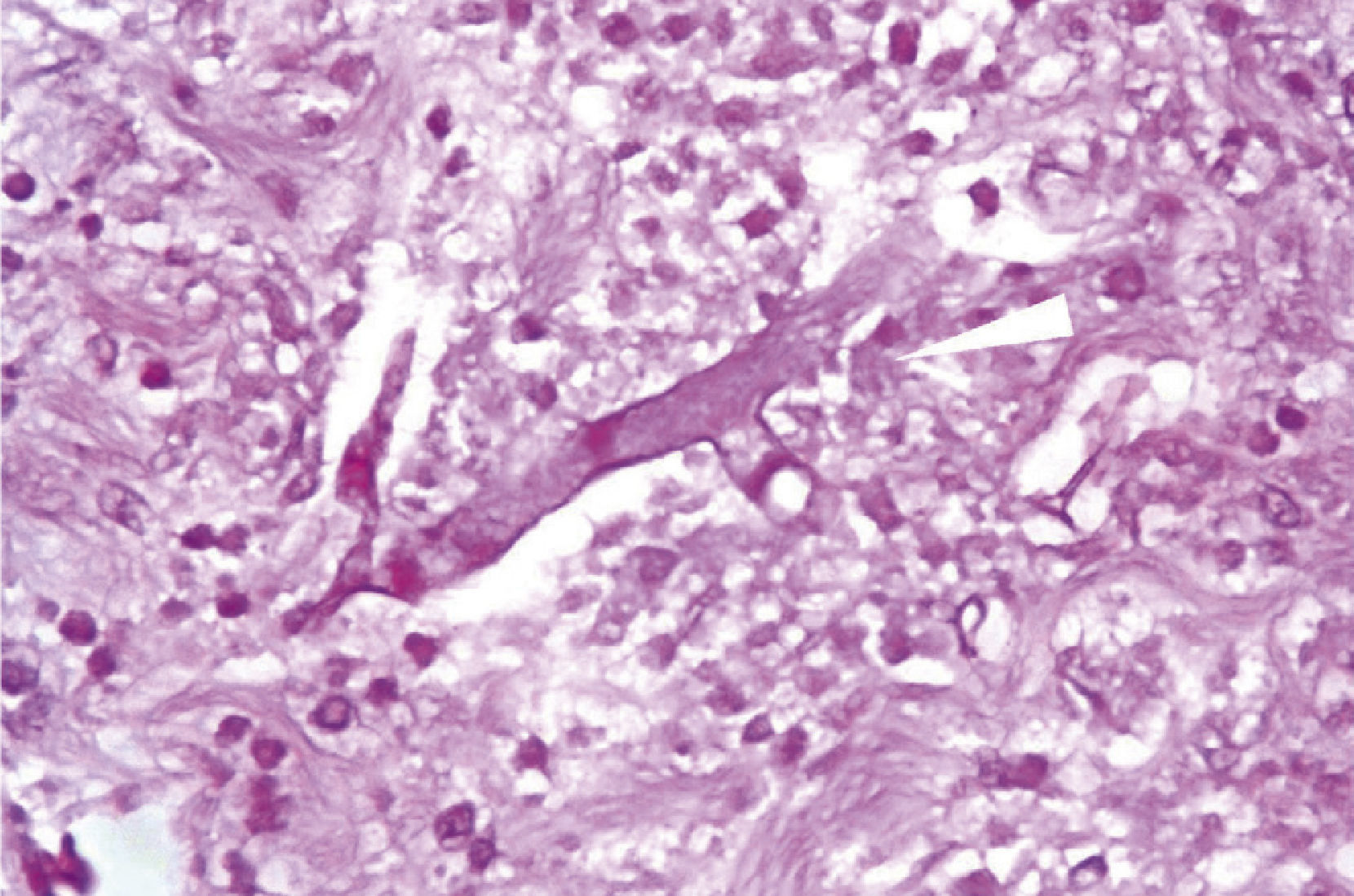
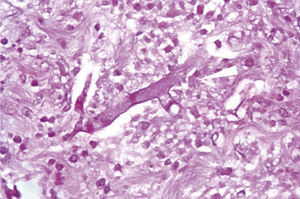
Microscopically, the presence of networks of aseptate hyphae in straight angle, secondary to mucormycosis fungal infection, with lesion-free surgical margins was observed (Fig. 4).
Initial treatment with amphotericin B at day 4 of admission at the ICU, with normal blood levels, no evidence of bleeding, haemodynamically stable, presenting reduction of aminergic support and biochemical improvement that allowed the patient to leave the ICU 18 days after surgery. Subsequently, the patient presented pulmonary condensation and pneumonia, confirming pulmonary mucormycosis by bronchial aspiration. Patient died from pneumonia complications 27 days after admission.
DiscussionMucormycosis is the rarest of the opportunistic fungi infections, such as those caused by Candida and Aspergillus spp. The annual incidence is calculated in 1.7 cases per million individuals, which amounts to approximately 500 cases per year in the United States. In a series of autopsies, the prevalence of mucormycosis ranges from 1 to 5 cases per 10,000 autopsies. This infection is 10 to 50 times less common than Candida or Aspergillus1 infection.
It is characterized by the presence of angioinvasion and thrombosis, and the consequent ischaemia and tissue necrosis, with the pathognomonic presence of networks of aseptate hyphae in straight angle in the tissues of a series of cases during biopsies or autopsies. The clinical presentations are rhino-orbital-cerebral (66%), pulmonary (16%), cutaneous (10%) and gastrointestinal (7%), involving the stomach (57.7%), colon (32.5%) and ileum (6.9%)10. Gastrointestinal mucormycosis is a rare infection, potentially fatal, that may be more frequent amongst the paediatric population and the complete digestive system may be involved3. It has been described in extremely undernourished patients (especially in children) and it is believed that it comes from the ingestion of the fungus. It has been observed in premature newborns associated with disseminated generalised disease. The most commonly involved places are the stomach, the colon and the ileum1,3. The report presented by Martinello et al.7 describes the presence of mucormycosis at the level of the jejunum in a patient with a history of malnutrition related to alcohol abuse and recent enteritis caused by Salmonella typhimurium, with the presence of acute abdomen, handled by surgery and medical treatment on the basis of amphotericin B and posaconazole, with a successful outcome3.
Acidosis interrupts the iron unions of the transferrin, increasing free iron, which probably promotes the growth of the fungi. In the study conducted by Turunc et al.4, none of the diabetic patients presented ketoacidosis, with a high mortality in the series of 11 patients who only had diabetes mellitus and chronic renal failure. The high mortality is attributed to both conditions. It is known that hyperglycaemia and acidosis affect neutrophil chemotaxis, and are responsible, as well as the phagocytes, for the elimination of mucorales fungi in patients with no alterations in the defence mechanism. Several factors have been found in our patient that may favour the mucorales fungi colonization, finding the perfect habitat for growth (ketoacidosis, hyperglycaemia).
The symptoms of the gastrointestinal mucormycosis are varied and depend on the place affected. The unspecified abdominal pain and distension associated with nausea and vomiting are common symptoms; fever and hematochezia may also occur7,10. It often starts with a diagnosis of intra-abdominal abscess. The diagnosis can be arrived at through biopsy of the suspected area during surgery or through endoscopy7.
In accordance with Jung et al.2, the emphysematous gastritis described by Frankel in 1889 is caused by the chronic consumption of alcohol, alkali or acids, gastric surgery, bacterial agents (E. coli, Clostridium welchi, Clostridium perfringens and S. aureus) and fungal agents (Pseudomonas, Aspergillus and mucormycosis), so the clinical manifestations are non-specific and the final diagnosis depends on the histological exam, which shows septa hyphae, infarction and angioinvasion.
Treatment is antifungal medicine and surgical debridement, as well as aggressive medical support for the comorbidities. It is known that due to the presence of necrosis in the tissues infected with mucormycosis, the antifungal agents penetrate them poorly, so it is necessary to administer a combined treatment10.
The delay in treatment with the amphotericin B based regimen (> 6 days after diagnosis) has been reported to be associated with twice the mortality at week 12. In patients with diabetes, with or without ketoacidosis, the efforts have to be put in restoring euglycemia and the normal acid-base state9.
In the case of our patient, the source of infection by fungal agent is unknown; although cases have been reported, as is the case in the series studied by Maraví-Poma et al.11, where gastrointestinal mucormycosis was transmitted by the use of wooden tongue depressor contaminated with angiospores at the Intensive Care Unit. Our patient was transferred from the Intensive Care Unit at the Hospital de Traumatología y Ortopedia; in addition to his comorbidities (type 1 diabetes mellitus, trauma), he also had ketoacidosis and other factors known as immunosuppressants. In their article, Jung et al.2 state that the coexistence of mucormycosis with the emphysematous gastritis may be the result of the presence of necrosis in the gastric wall caused by a bacterial infection or, alternatively, an invasion of mucormycosis may be possible, with the subsequent necrosis in the gastric wall that allows a secondary bacterial invasion and the production of intramural gas. A report describes that the most commonly associated condition is the presence of a pre-existing gastric ulcer, and the possibility that the gastric ulcer base denuded in an immunocompromised host may allow bacteria and fungi to invade deeply, leading to necrosis of the gastric wall and to the production of intramural gas 8. Martinello et al.7 describe a series of 66 cases with gastrointestinal mucormycosis disseminated to non-contiguous organs in 38% of the cases, and a high mortality related to intestinal perforation and to the upper digestive tract bleeding. In our case, it is believed that the primary place of the infection was the digestive tract with the subsequent dissemination to other organs, and being a patient who stayed in the ICU and who underwent invasive management, the contamination towards other organs may have been the cause of inoculation in other places, given the immunocompromised condition. When admitted to our unit, the patient presented pneumonia symptoms, in addition to digestive tract bleeding, and for this reason it could not be affirmed that it was secondary to inoculation by bronchoaspiration or by the contaminated equipment to which this type of patients were exposed. The patient was not submitted to necropsy because the family did not authorize it, thus it cannot be confirmed if there was infection in other intra-abdominal and/or extra-abdominal places.
ConclusionWe presented the case of a 32-year-old male with gastric mucormycosis, diagnosed before death, who, despite the combined medical and surgical treatment, presented fungal septicaemia with pulmonary dissemination and fatal outcome.
The diagnosis of an infection caused by mucorales requires a high degree of suspicion and since in the medical literature there are few cases reported, we are not very familiarized with that pathology, so it is an incidental finding for the digestive tract bleeding protocol and the corresponding indication of surgical intervention due to the deterioration of the patient. The success of treatment depends on early diagnosis, control of the risk factors, reduction of the immunosupression, and the quick polyene-based antifungal therapy, such as amphotericin B and liposomal amphotericin, as well as quick surgical debridement. It requires a high degree of suspicion because of its high mortality due to the fast progression of the disease in a patient with alterations in the immune system, which is more and more common in our society.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Alvarado-Lezama J. et al. Gastritis enfisematosa secundaria a mucormicosis gástrica. Cirugía y Cirujanos. 2015; 83: 56-60.