Myocardial noncompaction of the left ventricle is a congenital cardiomyopathy characterised by left ventricular hypertrabeculation and prominent intertrabecular recesses. The incidence ranges from 0.15% to 2.2%. Clinical manifestations include heart failure, arrhythmias, and stroke. Prognosis is fatal in most cases. Heart transplantation is a therapeutic option for this cardiomyopathy, and few had been made worldwide.
Clinical caseThe case is presented of a 20 year-old male with noncompacted myocardium of the left ventricle, who had clinical signs of heart failure. His functional class was IV on the New York Heart Association scale. He was successfully transplanted. Its survival to 15 months is optimal in class I New York Heart Association, and endomyocardial biopsies have been reported without evidence of acute rejection.
ConclusionIt is concluded that heart transplantation modified the natural history and improved survival in patients with this congenital heart disease.
El miocardio no compactado del ventrículo izquierdo es una miocardiopatía congénita caracterizada por hipertrabeculación del VI y prominentes recesos intertrabeculares. La incidencia oscila entre 0.15% a 2.2%. Las manifestaciones clínicas son: insuficiencia cardiaca, arritmias y embolias. Su pronóstico es mortal en la mayoría de los casos. El trasplante cardiaco es una opción terapéutica para esta miocardiopatía, y pocos han sido realizados a nivel mundial.
Caso clínicoVarón de 20 años con miocardio no compactado del ventrículo izquierdo que presentó datos clínicos de insuficiencia cardiaca en clase funcional IV de la Asociación Cardiológica Neoyorquina, y fue trasplantado en forma exitosa. Su sobrevida a los 15 meses es óptima en clase funcional I de la Asociación Cardiológica Neoyorquina y las biopsias endomiocárdicas se han reportado sin datos de rechazo agudo.
ConclusiónEl trasplante cardiaco es una opción terapéutica que modifica la sobrevida para este tipo de casos.
Myocardial noncompaction of the left ventricle (MNCLV) is a congenital and genetic myocardial disease characterised by hypertrabeculation of the left ventricle (usually the smooth endocardium), prominent intertrabecular recesses that receive blood flow directly from the left ventricular cavity rather than the coronary arteries, and coronary anomalies.1,2 It is believed that it is caused by noncompaction of the left ventricle during the 5th to 8th weeks of gestation.3
The incidence ranges from 0.15% to 2.2%, although in recent series with better diagnostic technology it has increased to 18%, especially if familial.4 MNCLV can be isolated, exclusive to the left ventricle, combined with the right ventricle or other cyanogen congenital heart diseases, or left or right ventricle outlet obstruction.1 In 1934, Bellet reported a case of congenital heart disease similar to MNCLV; Engberding published a similar case in 1984,5 and the first broad series of 8 cases was brought to the scientific world by Chin in 1990.6 It is, therefore, a recent disease categorised by the World Health Organisation since 1996 as an unclassified cardiomyopathy.7 Since 2006, the American Heart Association (AHA) has categorised the condition as a congenital heart disease.8,9
Clinical manifestations are principally heart failure (53%), arrhythmias that can be fatal (41%) and stroke (24%).10 Up to 82% of cases are accompanied by neuromuscular symptoms or manifestations.2 Manifestations can start from the second decade of life; principally heart failure due to dilation of the left ventricle. The natural progression of the disease can be death due to heart failure or fatal arrhythmias.10,11
Diagnosis is made by echocardiography, using the criteria of Chin6 and Jenni (2001).12
Because this diagnosis has a poor prognosis and there is a risk of sudden death and heart failure, one of the treatments indicated is placement of an automatic implantable defibrillator13 and heart transplantation.14,15
There are few cases in the literature on treatment of MNCLV by heart transplantation; the majority are for severe heart failure due to dilation of the left ventricular cavity.
On this occasion, we report a case of MNCLV after heart transplantation and their current progress.
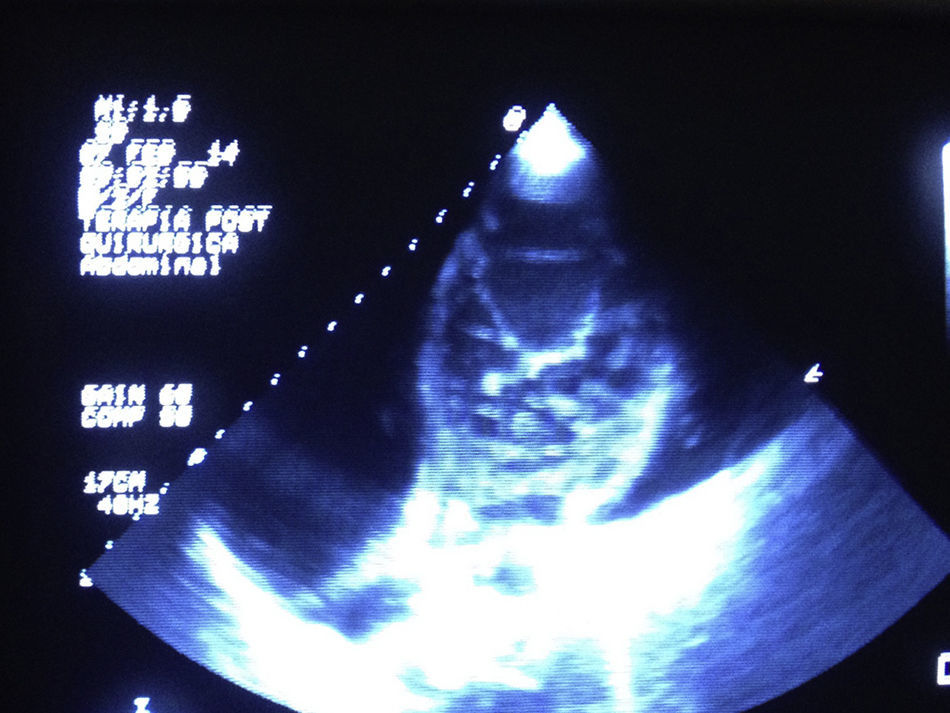
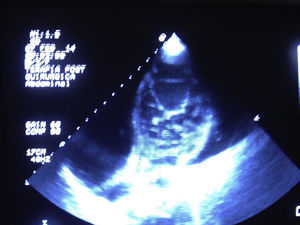
Clinical caseA 20-year-old male student with no relevant history and neurologically intact. The patient presented with symptoms of severe heart failure from August 2013, manifested by fluid retention, leg oedema, progressive dyspnoea on little exertion, orthopnoea and paroxysmal nocturnal dyspnoea, and heart arrhythmias. Treatment was started with amiodarone, diuretics and angiotensin-converting-enzyme Inhibitors. ECG showed hypertrabaculations on the lateral, apical and inferior surface of the left ventricle, relating to non-compacted myocardium with compacted epicardium higher than 2, occupying 21% of the ventricular surface (Fig. 1), diastolic diameter of 91mm, and left ventricular ejection fraction of 20% with grade II diastolic dysfunction. Coronarography showed no evidence of significant obstructive lesions. Pulmonary arterial trunk pressure was 42/10/21mmHg with pulmonary artery wedge pressure 11mmHg, transpulmonary gradient 10mmHg and pulmonary vascular resistance 2.1 Wood units.
With these signs, the patient was accepted for orthotopic heart transplantation which was performed on 22 February 2014, using the bicaval technique, with a cardiopulmonary bypass time of 124min and total ischaemia of 225min. The patient made good progress postoperatively. Mechanical ventilation was discontinued 6hours postoperatively. The patient was discharged from intensive care after 4 days and discharged home on the 10th postoperative day. He is now in his 15th month post heart transplantation, in functional class I, grafted heart with diastolic diameter of the left ventricle: 38mm and left ventricular ejection fraction of 68%. Endomyocardial biopsies show no evidence of rejection.
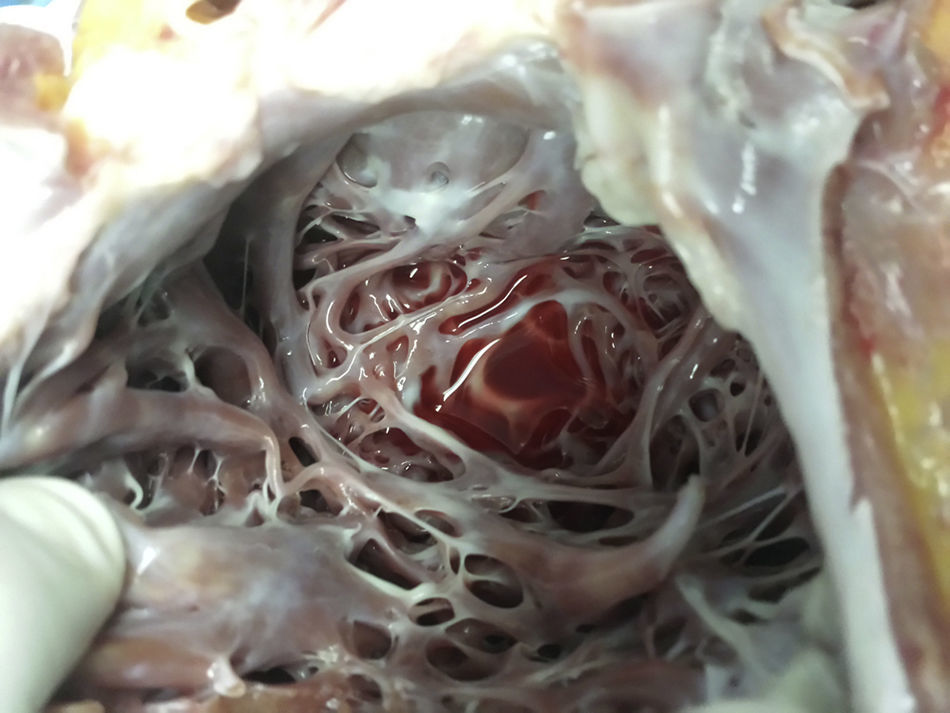
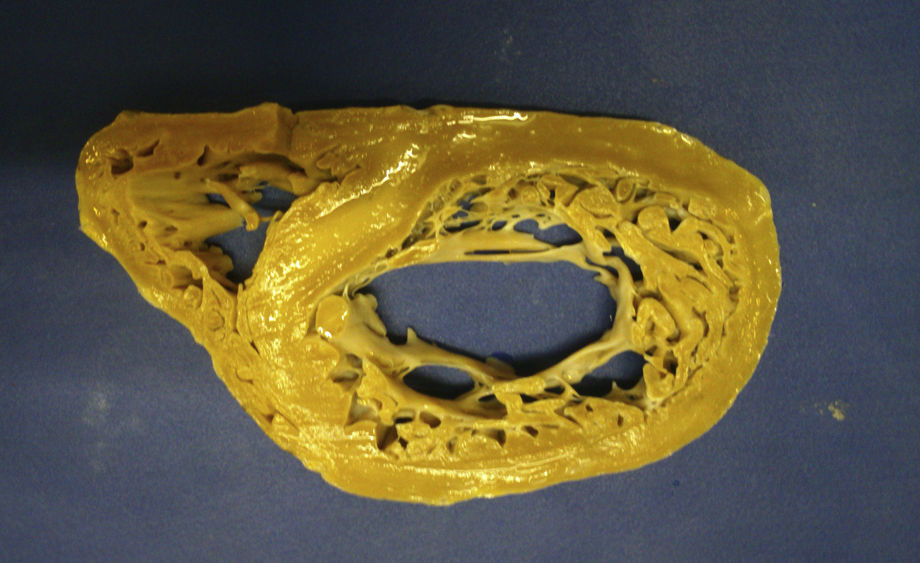
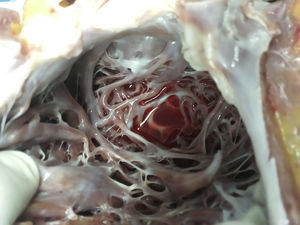
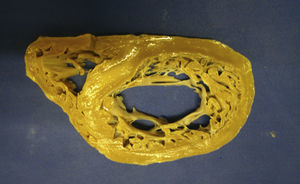
The macroscopic anatomopathological study of the removed heart was 450g in weight, thickened LV myocardial wall, the compacted portion measured 0.7mm and the non-compacted part 1.5mm, dilation of the left ventricular cavity, trabeculations and intertrabecular recesses in the apical, lateral and inferior left ventricle (Figs. 1–3). The microscopic report was fibrosis of the endocardium, myocardium with patches of eosinophils in the trabeculae, hypertrophy of the myocytes, some atrophied, and fibrosis of the interstitium.
DiscussionMNCLV is a congenital, heterogeneous, autosomal dominant heart disease that predominates in males. This cardiomyopathy can be associated with genetic disorders, such as: mutation of gene 4.5, located in the X chromosome, a mutation in the gene encoding the cytoskeletal protein, alpha-dystrobrevin (P121L), and with CYPHER/ZASP, E101K ACTC mutation and chromosome 11p15 locus.16 MNCLV is characterised by noncompaction of the left ventricle between the fifth and eighth weeks of gestation, probably because the intracavitary pressure failed to rise, which results in enormous trabeculations inside the LV (normally smooth) and the presence of intertrabecular recesses that communicate with the cavity of the left ventricle from which they receive blood flow rather than from the coronary arteries.17 Noncompacted cardiomyopathy usually exclusively affects the left ventricle but can include the right ventricle,18 it can exclusively affect the right ventricle and it can be accompanied by anomalies of the left or right ventricle outlet, valve disease, and restrictive and hypertrophic cardiomyopathies.19 Neurological manifestations present in 82%, therefore a thorough examination is necessary to rule out these disorders.2 There is a familial form of this cardiomyopathy, with a 50% chance of inheritance. Our case was exclusively MNCLV and not accompanied by other neurological disorders.
The clinical manifestations are severe heart failure (most frequent) due to cardiac dilatation and systolic and diastolic dysfunction of the left ventricle, arrhythmias from atrial fibrillation, ventricular tachycardia, ventricular tachycardia, intraventricular conduction disorders and embolic syndromes.20 The onset of clinical syndromes is between the second and fourth decades of life. the pathophysiology is due to hypertrophy of the noncompacted parts which causes areas of ischaemia and scarring, producing remodelling, dilation of the left ventricular cavity, systolic and diastolic dysfunction (probably due to cicatricial fibrosis)21 and arrhythmogenic areas; the blood flow will produce an embolic environment in the intratrabecular recesses. The apical, lateral and inferior regions of the left ventricle are most commonly noncompacted.22 Our case presented dilation of the left cavities, left ventricular ejection fraction (LVEF) of 20%, severe heart failure, functional class IV of the New York Heart Association, and left branch block.
The diagnosis was reached principally by echocardiography, and using the criteria of Jenni and Chin6,12; contrast medium to determine the endothelial area and the trabeculae provides greater sensitivity. Three-dimensional echocardiography was also performed with good results.23 In the event of any doubt, nuclear magnetic resonance has increased sensitivity and specificity for diagnosis. As we can see in the images this clinical case combines the echocardiographic criteria of Chin and Jenni.6,12
The prognosis varies from one series to another, with deaths in up to 60%, principally sudden death due to fatal arrhythmias, severe heart failure and embolic events. The variables with the poorest prognosis are dilation of the left ventricular cavity, LVEF ≤35%, New York Heart Association functional class III–IV, persistent atrial fibrillation and left branch block, and fatal arrhythmias. The therapeutic options for these adverse factors are AID placement, anticoagulation and heart transplantation.11
Only 11 cases of MNCLV undergoing heart transplantation had been reported up until 2010. This cardiomyopathy is rare and reports of heart transplantation are rarer still.24,25 The cases were secondary to acute severe heart failure. There are no medical reports in Latin America of transplantation due to MNCLV. Val Bernal reported one case in Spain in 2006.26 Our case is a 20-year-old male patient who underwent a successful heart transplantation, and 15 months later is functional class I, with heart biopsies that show no evidence of acute rejection and a normally functioning grafted heart, with a LVEF of 65% and pulmonary arterial pressure 35/12 (24mmHg).
ConclusionMNCLV is a rare congenital cardiomyopathy, with 3 manifestations: severe heart failure, fatal arrhythmias and embolic phenomena, with a prognosis of sudden death as high as 60%. Heart transplantation is a good option to treat these cases, after careful selection of the recipient.
Ethical responsibilitiesProtection of people and animalsThe authors declare that neither human nor animal testing have been carried out under this research.
Data confidentialityThe authors declare that they have complied with their work centre protocols for the publication of patient data.
Privacy rights and informed consentThe authors declare that no patients’ data appear in this article.
Conflict of interestsThe authors have no conflicts of interests to declare.
Please cite this article as: Hugo Jesús Zetina-Tun HJ, Careaga-Reyna G, Galván-Díaz J, Sánchez-Uribe M. Trasplante cardiaco: una opción para tratamiento del miocardio no compactado aislado de ventrículo izquierdo. Primer caso en México. Cir Cir. 2017;85:539–543.