The presence of multiple symptomatic pulmonary nodules and one cardiac tumour in a child requires urgent diagnosis and treatment. Until a few decades ago, the diagnosis of a cardiac tumour was difficult and was based on a high index of suspicion from indirect signs, and required angiocardiography for confirmation. Echocardiography and other imaging techniques have also helped in the detection of cardiac neoplasms. However, it is not always easy to make the correct diagnosis.
Clinical caseThe case is presented of a 12-year-old boy with pulmonary symptoms, and diagnosed with a cardiac tumour with lung metastases. The presence of numerous pulmonary nodules was confirmed in our hospital. The echocardiogram detected a solid cardiac nodule in the right ventricle. Magnetic resonance imaging confirmed the findings and the diagnosis. Puncture-aspiration of a lung nodule gave the diagnosis of hydatidosis. He underwent open-heart surgery with cardiac cyst resection and treated with anthelmintics. The lung cysts were then excised, and he recovered uneventfully.
DiscussionThis child had multiple pulmonary nodules and a solid cardiac nodule, and was suspected of having a cardiac tumour with pulmonary metastases. However, given the clinical history, background and morphology of pulmonary nodules, another possible aetiology for consideration is echinococcosis. The clinical picture of cardiac hydatidosis and its complications is highly variable. The clinical history is essential in these cases, as well as having a high index of suspicion.
ConclusionHydatidosis should be included in the differential diagnosis of a solid, echogenic, cardiac nodule. The treatment for cardiopulmonary hydatid cysts is surgical, followed by anthelmintics.
La presencia de múltiples nódulos pulmonares sintomáticos y uno cardíaco en un niño exigen un diagnóstico y tratamiento urgentes. El diagnóstico de una neoformación cardíaca era difícil hasta hace pocas décadas, y se basaba en un alto índice de sospecha ante signos indirectos, necesitando la angiocardiografía para su confirmación. La ecocardiografía y otros medios de imagen han facilitado la detección de los nódulos cardíacos. Sin embargo, no siempre es fácil acertar con el diagnóstico.
Caso clínicoNiño de 12 años con síntomas pulmonares. Diagnosticado de tumor cardíaco con metástasis pulmonares. En nuestro hospital se confirmó la presencia de numerosos nódulos pulmonares. El ecocardiograma detectó un nódulo cardíaco sólido ventricular derecho. La resonancia magnética nuclear confirmó los hallazgos, haciéndose el mismo diagnóstico. La punción-aspiración de un nódulo pulmonar fue diagnóstica: hidatidosis. Fue operado a corazón abierto resecando el quiste cardíaco y tratado con antihelmínticos. Posteriormente se extirparon los quistes pulmonares. Se recuperó el paciente sin complicaciones.
DiscusiónEn este niño, con múltiples nódulos pulmonares y uno cardíaco sólido, se hizo el diagnóstico de tumor cardíaco con metástasis pulmonares; sin embargo, con la historia clínica, los antecedentes y la morfología de los nódulos pulmonares se debió incluir la equinococosis como posible etiología. El cuadro clínico de los quistes hidatídicos cardíacos y de sus complicaciones es muy variable. En estos casos es fundamental la historia clínica y tener un alto índice de sospecha.
ConclusiónEn el diagnóstico diferencial de un nódulo cardíaco sólido, ecodenso, debe incluirse la hidatidosis. El tratamiento de los quistes hidatídicos cardiopulmonares es la cirugía.
Premortem diagnosis of a cardiac neoformation was very difficult up until a few decades ago.1,2 The two-dimensional echocardiograph has made it possible to detect these tumours.1 Nuclear magnetic resonance (NMR) enables better definition of their morphology and location. However, with these imaging tests it is not always easy to reach a correct diagnosis.
Neoplasms are the most frequent cause of the appearance of simultaneous nodules in the heart and lung. Primary cardiac tumours in children are rare and only from 8% to 10% are malignant.3,4 Myxoma is rare in children1,4 and is the only benign tumour to produce embolisms, its location in the right ventricle embolising the lungs is very rare.5–7 Malignant primary cardiac neoplasms, which can develop in the right ventricle and cause pulmonary metastases, include rhabdomyosarcoma and angiosarcoma, which are rare in childhood.3,8,9 Malignant cardiac tumours secondary to metastases of cancers in other sites are 20–40% more common than primary cardiac tumours.3,9
Hydatid cysts can implant in the heart2,10,11 and give solid images on ultrasound, simulating tumours.12–15 Cysts located in the right ventricle can cause embolism and pulmonary dissemination.13
The objective of this article was to present the case of a child found to have multiple pulmonary nodules and one solid echodense cardiac nodule, and to review the errors committed in the initial diagnosis and the surgical treatment once the aetiology of the lesions had been confirmed.
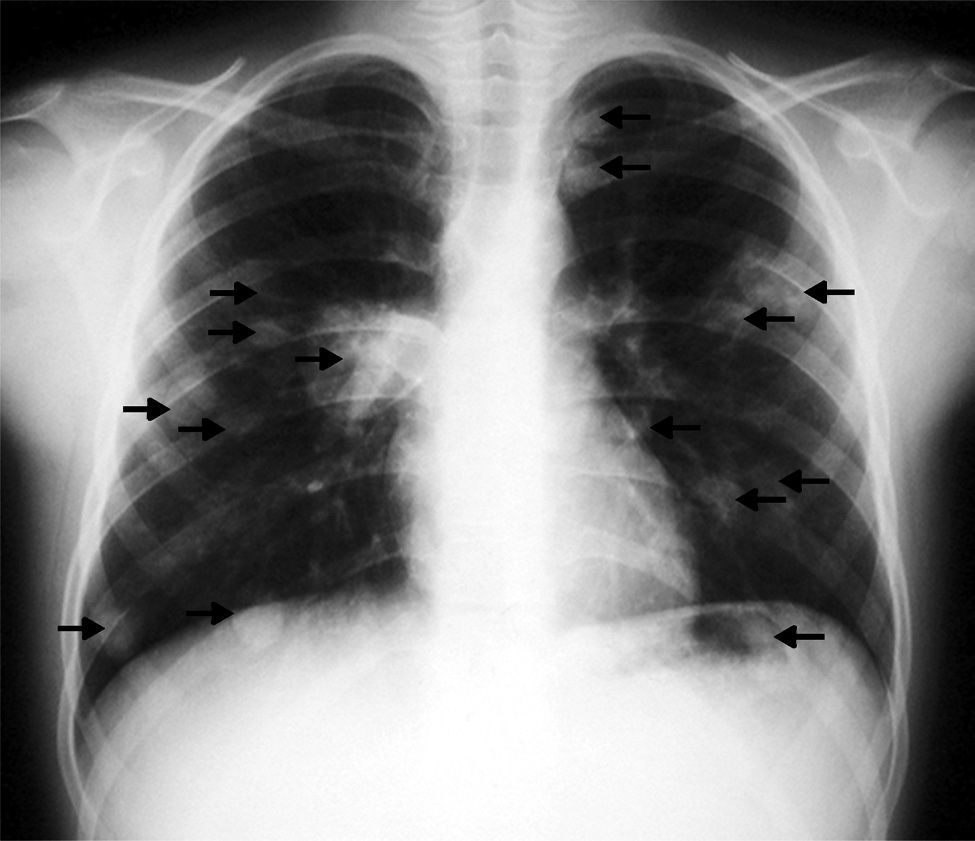
Clinical caseA 12-year-old Romanian boy, who had been resident in Spain for a year and a half, attended the Emergency Department presenting with: a productive cough, occasionally with bloody sputum, and pain in the left hemithorax (attributed to a fall from a tree); in the days prior to the consultation he had presented a fever. Physical examination was normal, apart from a temperature of 39.5°C. The laboratory tests showed moderate leukocytosis, blood cultures were negative, as were the tests for tuberculosis and fungal infections. Chest X-ray showed multiple nodules in both lungs. Computed axial tomography (CAT) of the chest revealed numerous nodules of different sizes in both lungs.
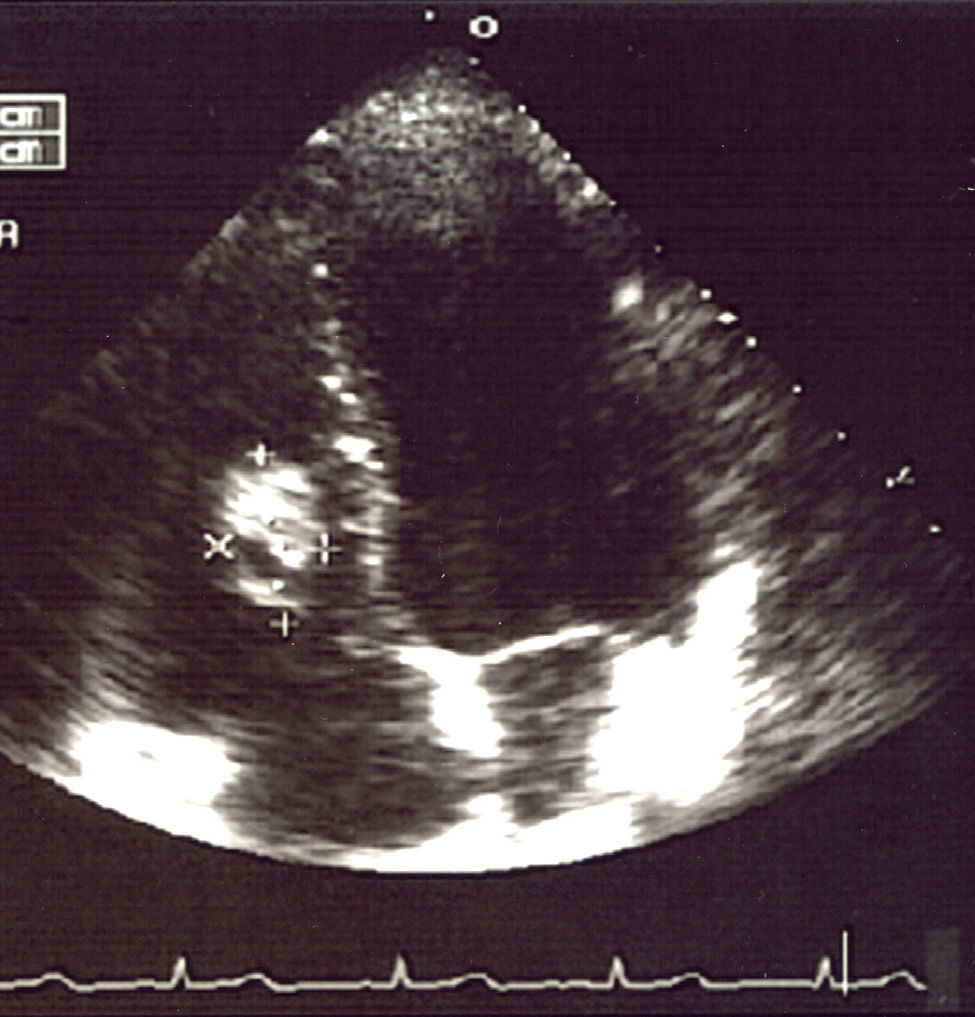
A fine needle puncture was performed on one of the nodules and the result was compatible with a non-specific inflammatory process. The echocardiogram showed a nodule of approximately 3cm in the right interventricular septum.
The patient was started on antibiotic treatment when a right-sided parahilar infiltrate appeared, along with the diagnoses of a cardiac tumour with pulmonary metastasis and associated pneumonia. For this reason, the patient was referred to the paediatric oncology department for study and treatment.
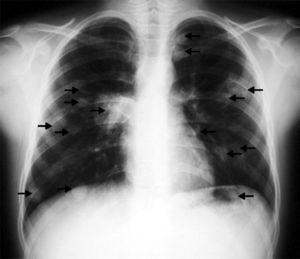
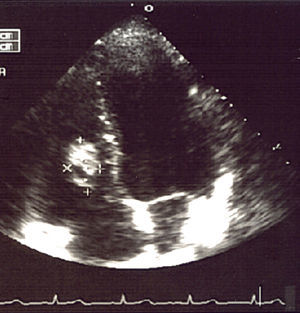
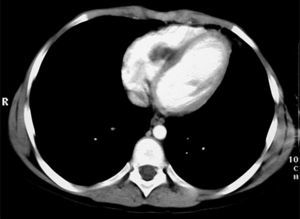
The tests performed gave similar results to the previous tests; chest X-ray showed multiple nodular images in both lungs (Fig. 1), and an area with increased density in the right parahilar region. The echocardiogram (Fig. 2) showed a nodule of greater echogeneity than the myocardium of 26×18mm, located on the right side of the interventricular septum affecting the anterior papillary muscle. Therefore, a differential diagnosis was established between sarcoma, myxoma, lymphoma and endocarditis vegetation.
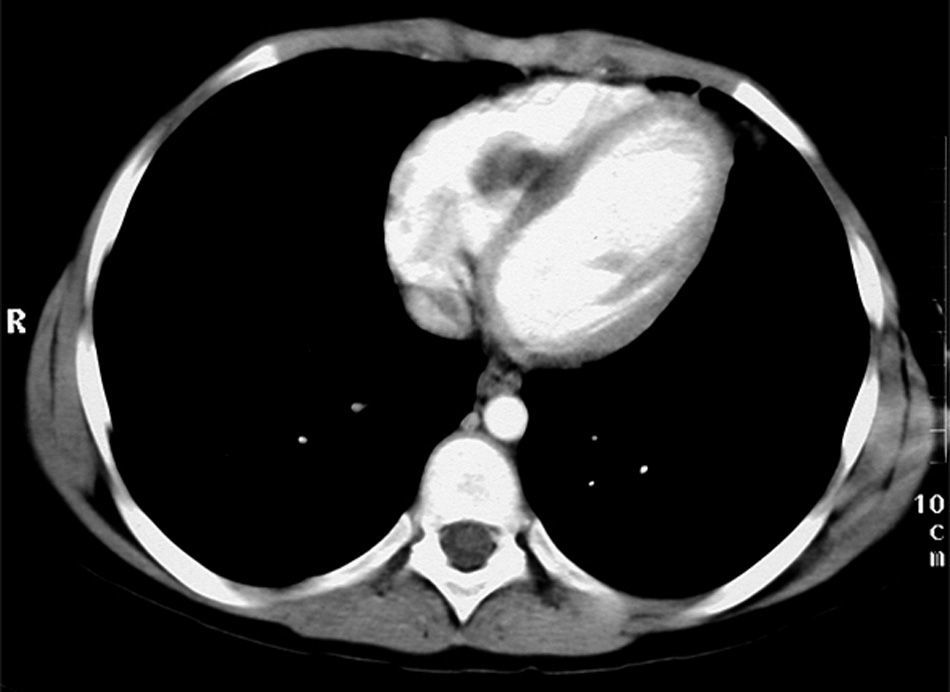
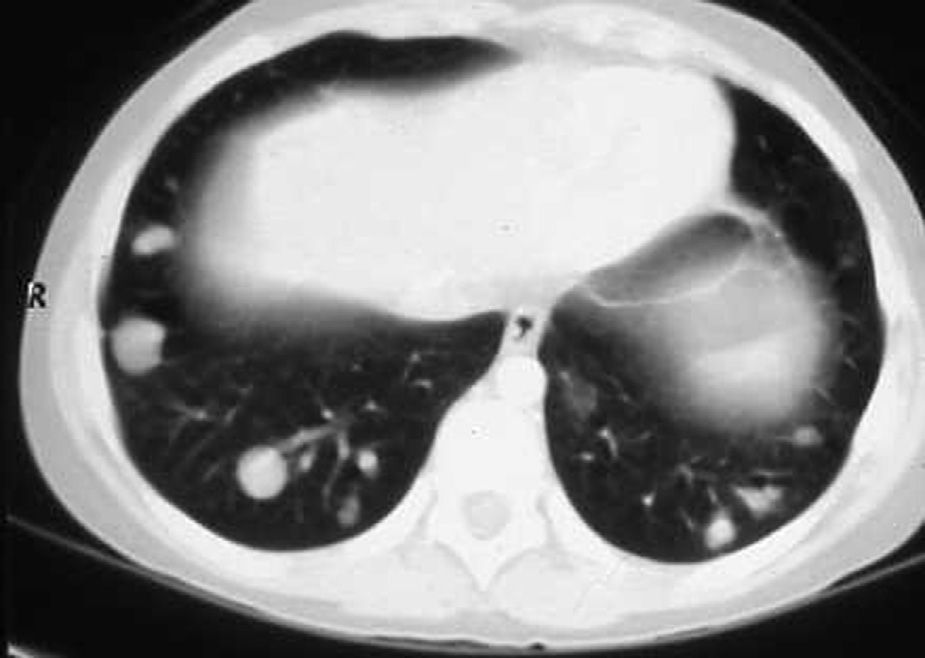
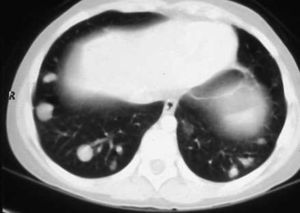
Cardiac NMR (Fig. 3) showed a nodule of 26mm in diameter in the right ventricle, adhering to the interventricular septum. NMR of the chest (Fig. 4) revealed multiple pulmonary nodules of different sizes predominating in the bases and pulmonary condensation in the upper right lobe. With a clinical diagnosis of a possible cardiac tumour with pulmonary metastasis, it was decided to biopsy one of the pulmonary nodules. Under tomographic control using CAT, an aspiration puncture was performed of a left pulmonary nodule without incident. The anatomopathological report revealed the presence of numerous tapeworms showing the heads and refringent hooks and intensely PAS positive chitinous membranes; a diagnosis of hydatidosis was made. A serology test was positive for echinococcosis. Albendazole was added to the antibiotic treatment. Once the lung infection had been resolved, surgical resection was planned, firstly of the cardiac nodule through a right ventriculotomy, given its inferior location and possible involvement of the anterior papillary muscle of the tricuspid valve.
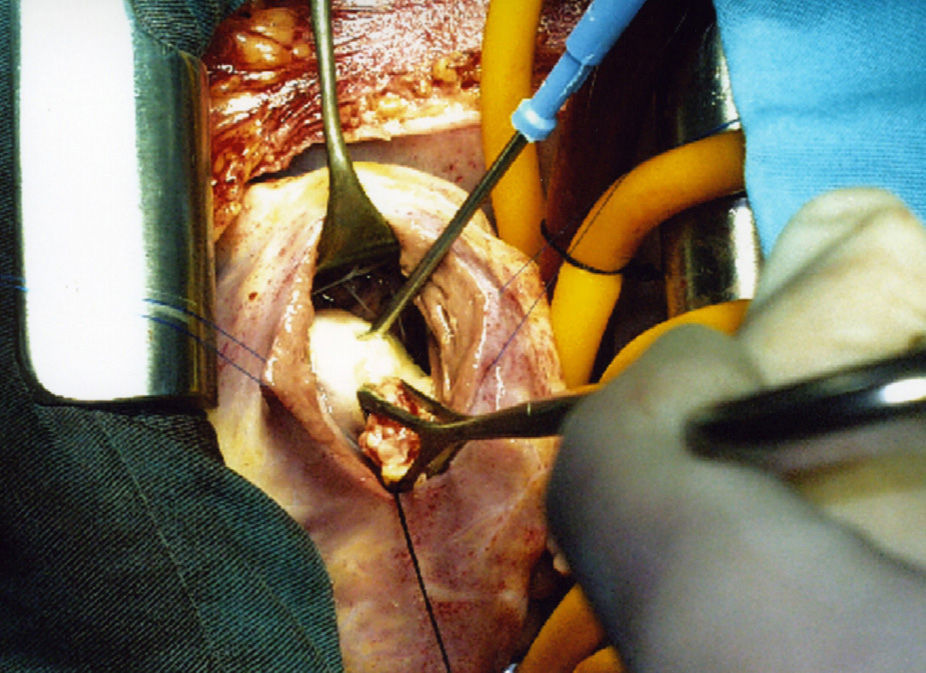
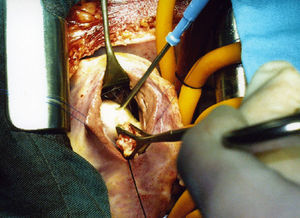
The surgical procedure took place under general anaesthetic with histamine receptor block. Mid sternotomy; under extracorporeal circulation and haematic cardioplegia, through a right transverse ventriculotomy, a puncture-aspiration was performed and hypertonic saline was injected into the cyst. The cyst was resected with curettage of the septum. A group of tendon cords from the septal valve of the tricuspid valve that had inserted in the upper pole of the cyst and were affected by the resection were reimplanted.
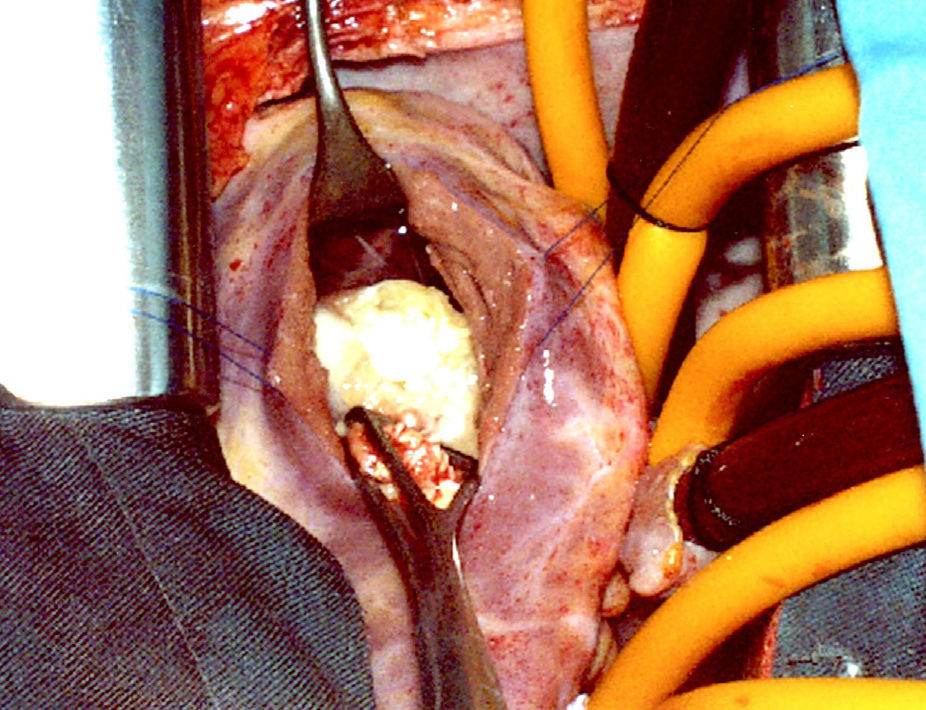
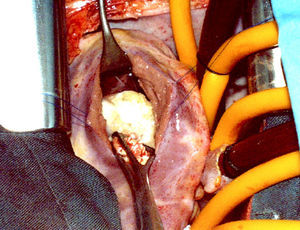
An ovoid formation 25×15mm was found in the lower part of the interventricular septum, limited by the supraventricular crest and the arcuate fasciculus, located below the anterior papillary muscle without affecting it (Fig. 5). It was pearly white in colour, of firm consistency in the lower 2/3, yellowish, soft granulomatous tissue projected in the upper part. Multiple small vesicles of 5–6mm, intensely white in colour and containing clear liquid were extracted from the inside of the cyst (Fig. 6). The nodule penetrated about 6mm into the interventricular septum. The final diagnosis was a fissured hydatid cyst.
The patient made excellent postoperative progress and was discharged from hospital in a good general condition, with albendazole treatment.
At 6-month follow-up, the echocardiograph was normal, with no tricuspid insufficiency and normal mobility of the interventricular septum. Six months after the cardiac operation, the left pulmonary cysts were resected through a thoracotomy. Fourteen were removed; one had ruptured into the airway in the upper lobe, making this an atypical resection at this level. Once month later, an operation was performed to remove numerous cysts from the right lung, 2 communicating into the airway. None of the pulmonary cysts contained live parasites.
The postoperative outcomes were good. At one-year follow-up after the lung interventions the patient remained asymptomatic, pleuropulmonary scarring sequelae were observed on the CAT scan, one nodule of 0.6mm in the left lung and other small residual nodules in the right lower lobe, present in the preoperative studies and there have been no changes since then.
DiscussionTumours which do not embolise and those that do not cause lung metastases should be excluded in the differential diagnosis of a solid, echodense intracavitary cardiac nodule accompanied by multiple pulmonary nodules. Primary cardiac tumours in childhood are rare and only 8–10% are malignant.3,4 The only benign cardiac tumour which leads to embolisms is myxoma, which is rare in children.1,4 In a series of 49 cardiac tumours, 33 myxomas were found, only 4 found in children with one in the right ventricle.5 Eighty six percent of myxomas are located in the left atrium, and 3% in the right ventricle.6,7
The clinical symptoms that the child presented were compatible with pulmonary embolism. However, the lung imaging tests ruled out this diagnosis. In this case, the morphology of the cardiac nodule, its immobility, its location and its co-existence with multiple spherical pulmonary nodules with no pulmonary hypertension excluded myxoma.
Of the primary malignant cardiac neoplasms in children, only rhabdomyosarcomas and angiosarcomas are located in the right cardiac cavities and progress to metastases or pulmonary embolisms. Rhabdomyosarcomas tend to grow in children's septums.8 They are fast to evolve and cause obstructive complications, and in 50% of cases pericardial invasion.3,8 Angiosarcomas are primary malignant cardiac tumours that are more common in adults and are rare in children, 80% are located in the right atrium causing pulmonary metastasis.3
These tumours are fast growing and induce cardiac failure and arrhythmias. They invade the pericardium causing blood leakage and are accompanied by a fever, anorexia and general deterioration. Their short-term prognosis is fatal.3,9 Our patient had no cardiac signs or symptoms, his general condition was good and the size of the cardiac nodule had not changed from the time it was discovered.
Cardiac neoplasms secondary to metastasis of malignant tumours in another location are 20–40% more common than primary malignant cardiac tumours.3,9 In this child no tumours had been detected, this ruled out the cardiac and pulmonary nodules as being metastatic. We ruled out multiple pulmonary embolisms with a thrombus retained in the right ventricle or endocarditic vegetation with pulmonary embolisms because there were no symptoms of dyspnoea, right heart failure or significant septic symptoms.
The clinical history from the original hospital mentioned: “The child has direct contact with sheep, goats and dogs”. It confirmed that his parents were shepherds and had also been shepherds in Romania, where the child grew up surrounded by sheep and dogs. With this background, hydatidosis should have been considered, especially from the lung images, because the cardiac nodule did not have the appearance of a cyst.
Echocardiography is essential in the diagnosis of cardiac hydatid cysts. Spanish authors are pioneers in the study of these cysts,10,11 but intracavitary cysts can appear solid and look like tumours and this makes an accurate diagnosis difficult.12–14 Oliver et al.11 studied 15 cases of cardiac hydatidosis finding this type of image in 4 septal cysts, similar to ours, 2 fissured with dissemination of echinococcosis. In our patient the fissuration of the cyst might have contributed towards giving the image of a solid nodule which was diagnosed as a tumour, as has happened in other cases. A female patient in the Mayo Clinic14 was diagnosed with cardiac angiosarcoma and the cyst was discovered during surgery. Berincioglu et al.15 published the case of a child diagnosed with a left ventricular tumour by echocardiography, CAT, NMR and transoesophageal echo; a diagnosis of hydatidosis was made after performing an intraoperative puncture-aspiration. For all these reasons, echinococcosis should be included in the differential diagnosis of a solid, echodense cardiac nodule.11,12,14,15
Hydatid cysts, principally caused by Echinococcus granulosus, are a common parasitosis in regions where there is abundant sheep farming. In Spain, where it was once endemic and control and prevention had been relaxed,16 it has reappeared with immigration.12 There are numerous publications on cardiac echinococcosis,10–12,17 of these, only 0.5–2% implant in the heart. The most common site is the left ventricle, followed by the interventricular septum;13,14 the cysts tend to coexist with cysts in other locations.2
Cardiac hyatidosis is rare in children.18 However cysts located in the right side of the heart have been associated with pulmonary embolism19,20 and pulmonary hydatidosis secondary to fissuration with dissemination of the parasite.13 These cysts can result in serious complications, which can even be fatal, such as anaphylaxis, arrhythmias and massive pulmonary embolism.2,13,20,21
The treatment of choice in intracardiac cysts is still surgical resection under extracorporeal circulation.2,13,21–23 Nonetheless, surgery can be extremely difficult, in García Ortiz’ case2 a cardiomyoplasty with a diaphragm was performed, in order to close the hole which resulted from the resection of the cyst. The histamine receptors need to be blocked in anaesthesia in order to prevent anaphylaxis. Steps should be taken to prevent the dissemination of the parasite during surgery. Puncture aspiration of the cyst followed by the injection of a scolicidal fluid before opening it helps to prevent the spread of live parasites, 20–30% saline is effective and does not damage cardiac tissue,13 since other fluids13,18 can cause serious damage. Ideally a pericystectomy should be performed, but depending on its location, it is wise to leave part of the pericystic fibrous tissue in order not to cause structural damage, atrioventricular block or interventrical communication in the septums.13,23,24 If the cardiac cyst is accompanied by other accessible pulmonary cysts these can be removed during the same operation.25,26 In our case, because the cysts were multiple and disperse, we chose to resect them in different operations. Surgical treatment should be complemented by the administration of albendazole in the post-operative period.
ConclusionsCardiac hydatidosis is rare and can simulate other diseases; therefore, cardiac tumours should be included in the differential diagnosis.
The treatment of cardiac hydatid cysts is open heart surgery, followed by the appropriate treatment with antihelmintics. In this case, the initial mistaken diagnosis confused the way the patient was managed. Had greater attention been paid to the patient's background in his clinical history and an appropriate differential diagnosis made that took into account other diseases, which are common in our environment, a correct initial diagnosis would have been reached.
Hydatidosis is a parasitosis that causes major complications, therefore great efforts are necessary to prevent it.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Martín-Izquierdo M, Martín-Trenor A. Hidatidosis simulando un tumor cardíaco con metástasis pulmonares. Cir Cir. 2016;84:318–323.