Tissue storage is a medical process that is in the regulation and homogenisation phase in the scientific world.
The international standards require the need to ensure safety and efficacy of human allografts such as skin and other tissues. The activities of skin and tissues banks currently involve their recovery, processing, storage and distribution, which are positively correlated with technological and scientific advances present in current biomedical sciences.
A description is presented of the operational model of Skin and Tissue Bank at Instituto Nacional de Rehabilitación as successful case for procurement, recovery and preservation of skin and tissues for therapeutic uses, with high safety and biological quality. The essential and standard guidelines are presented as keystones for a tissue recovery programme based on scientific evidence, and within an ethical and legal framework, as well as to propose a model for complete overview of the donation of tissues and organ programmes in Mexico. Finally, it concludes with essential proposals for improving the efficacy of transplantation of organs and tissue programmes.
El almacenamiento de tejido es un proceso médico en fase de regulación y homogenización científica en el mundo. Los estándares internacionales exigen garantizar la seguridad y la eficacia de los aloinjertos humanos como piel y otros tejidos. En la actualidad las actividades de los bancos de piel y tejidos involucran la recuperación, procesamiento, almacenamiento y distribución como proceso de desarrollo, que se correlaciona positivamente con los avances tecnológicos y científicos presentes en las ciencias biomédicas actuales.
Se describe el modelo instaurado por el Banco de Piel y de Tejidos del Instituto Nacional de Rehabilitación como un caso exitoso para la procuración, recuperación y preservación de piel con fines terapéuticos, alta seguridad sanitaria y elevada calidad biológica. Se discuten los fundamentos y estándares empleados en el programa actual de recuperación de tejidos con base en la evidencia científica disponible, el contexto ético y el marco jurídico vigente de la donación de tejidos en México. Se concluye con algunas propuestas para mejorar la eficacia de los programas de trasplantes.
Although several primitive attempts to carry out transplants were rudimentarily described almost 2500 years ago, in the so-called “Sutrasthanam manuscripts”,1 where they appeared as an incipient surgical procedure against the damage caused to the bodies of soldiers, it was not until the beginning of the 20th century that the first documented processes were reported regarding the use of tissues preserved at low temperatures over several days and subsequently replanted into the same donor.2
The Luyet3 and Webster4 studies documented freezing as an effective method for the temporary preservation of tissues. In accordance with Wright et al.5 Falt and Marragonni reported the first storage procedures of skin from dead bodies using solutions supplemented with 10% serum as a preserving agent.5,6 All these steps led to the creation of a new type of establishment from the end of the 1980s, equipped to store skin and other tissues. These installations were latterly known by their generic name as “skin banks”.
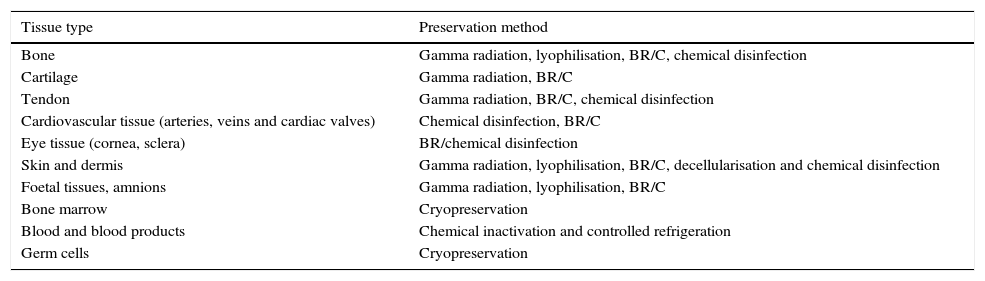
The use of human allografts is closely related to the development of different methods for the preservation of tissues (Table 1). These strategies are designed to integrate tissue recovery procedures which seek: (1) cellular viability maintenance7; (2) the preservation of proteins8; (3) the presence of growth factors such as: the epidermal growth factors, the vascular endothelial growth factor, the transforming growth factor beta and pro-inflammatory cytokines (Il-2, Il-6, Il-10 TNF-α alfa); and (4) the complete integrity of tissues. All these factors focus on generating biological products of high quality, with top health safety and of extreme therapeutic value.9
Methods for tissue preservation for transplant uses.
| Tissue type | Preservation method |
|---|---|
| Bone | Gamma radiation, lyophilisation, BR/C, chemical disinfection |
| Cartilage | Gamma radiation, BR/C |
| Tendon | Gamma radiation, BR/C, chemical disinfection |
| Cardiovascular tissue (arteries, veins and cardiac valves) | Chemical disinfection, BR/C |
| Eye tissue (cornea, sclera) | BR/chemical disinfection |
| Skin and dermis | Gamma radiation, lyophilisation, BR/C, decellularisation and chemical disinfection |
| Foetal tissues, amnions | Gamma radiation, lyophilisation, BR/C |
| Bone marrow | Cryopreservation |
| Blood and blood products | Chemical inactivation and controlled refrigeration |
| Germ cells | Cryopreservation |
BR/C, biological recovery and cryopreservation.
The procurement and preservation of tissues is a rapidly and increasingly developed activity which is positively correlated with technological and scientific advances present in biomedical sciences during the 20th century. Furthermore, demand for allografts for therapeutic reasons is projected to increase considerably as a result of the increase in life expectancy and its concomitant increase in chronic degenerative diseases. The design of successful institutional strategies capable of promoting donation activities and tissue retrieval10–12 has therefore become imperative.
The aim of our study consisted of describing the current panorama of tissue retrieval and storage with the design of a prototype institutional model authorised by the Banco de Piel y de Tejidos del Instituto Nacional de Rehabilitación (INR) for retrieval and storage of high therapeutic value, thanks to the use of scientific principals applied to self sustainability of the health system needs in Mexico.
Worldwide regulation of skin banksSeveral international associations which promote protocols and directives for the technical operation of skin and tissue banks13–16 were created in order to organise establishment and operations. The following are of note: (1) American Association of Tissue Banks, created in 1972; (2) Euroskin Bank (Bewerjwick, NL) founded in 1976 and called Tissue Bank from 2010; (3) European Association of Tissue Banks, established in 1991; (4) Asociación Española de Bancos de Tejidos, founded in 2002; (5) Asociación de Bancos de Tejidos de Asia-Pacífico; and (6) la Asociación Latinoamericana de Bancos de Tejidos.
All these organisations try to regulate the standardisation of directives for the therapeutic storage and use of tissues for transplant, in conjunction with other governmental, ethical and scientific bodies. The following central points may be highlighted from the international standards: (1) ethical aspects regarding the procurement of tissues based on altruism and not trading; (2) regional regulation of prevailing health laws; (3) administrative organisation; (4) standards for physical installations of tissue banks; (5) recuperation of tissues with pharmaceutical quality and standards; and (6) traceability of preservation processes.
Tissue banks in MexicoIn the Mexican Republic initial activities related to tissue storage were made with bone material. Pioneering procedures took place in the Hospital Central Militar and the Hospital Infantil de México in 1944. During the 1950s similar activities were initiated in Hospital Rubén Leñero and in Hospital Regional de Monterrey.
In 1997 the Hospital Central de Petróleos Mexicanos and the Universidad Nacional Autónoma de México carried out several procedures relating to tissue storage, using the then available international standards. The Banco de Tejidos of the Instituto Nacional de Investigaciones Nucleares (ININ) is prominent here as it began to use gamma radiation to sterilise bone tissues, pig hide and amniotic membranes.
Activities in Skin and Tissue Banks today involve the procurement, processing, storage and distribution of biological products classified by the current Ley General de Salud as “biological supplies” as they include an industrialisation process which guarantees their safety and efficacy.
On 30th January 2013 the national register of the Centro Nacional de Trasplantes recorded 56 establishments classed as Banks, which were regulated jointly with the Comisión Federal de Protección contra Riesgos Sanitarios (COFEPRIS).
Legal framework of tissue banks in MexicoThe Sistema Nacional de Trasplantes (SNT) is a structure located within the Sistema Nacional de Salud, consisting of all the establishments and hospitals possessing a licence to carry out organ or tissue donor transplant activities and cell or tissue bank activities authorised by COFEPRIS. All these bodies are subject to the regulations established by law and specifically that laid down in Title XIV of Ley General de Salud, in the legal regulations issued on these matters and other directives.13
In compliance with prevailing legislation, the Skin and Tissue Bank of the Instituto Nacional de Rehabilitación is authorised to offer organs, tissues and cells for transplant, through organ, tissue extraction, skin and muculoskeletal tissue transplant, and also a tissue bank (skin and musculoskeletal tissue). The legal context enables the Skin and Tissue Bank to have an innovative and far-reaching approach in its procedures model for procurement, preservation and transplant of tissues for therapeutic purposes. This approach includes social aspects for the promotion of an organ and tissue donor culture and the training of highly specialised human resources in donor and tissue preservation matters.
The transplant Committee as a regulating body of Mexican bank activitiesThe corner stone of the SNT are the Internal Transplant Committees in each hospital. In compliance with article 316 of the Ley General de Salud, these collegiate bodies comprise a health care director and a group of health professionals who are experts in this sector, coordinators in donation and transplant, and the necessary administrative support staff for these procedures.13,17
Although in the strict sense of the word, the main function of a bank is to safeguard tissues until use, this activity should be secondary to the development of preservation activities with traceability of transparency and safety for operators and users. The supervision of these activities was entrusted to the Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS).
Problems with skin and tissues banks in MexicoDue to the existing lag in adopting this type of establishment the following problems arise in Mexico: (1) there is little experience in the practise of tissue storage; (2) a high learning curve exists and high costs associated with specific training in this area; (3) there is no active training and educational programme for the culture of organ and tissue donation; (4) no integrated model of donation, procurement and cadaveric skin processing exists within the Sistema Nacional de Salud; (5) there is very little financial investment in scientific research aimed at tissue storage; (6) there are problems derived from current legislation and classification of biological tissues as “supplies” in the Ley General de Salud; (7) private companies are competing for tissue donation; (8) the health sector lacks any industrial development for the generation of supplies; (9) ethical problems arise derived from private banks competing for donors, through practices which lack sufficient regulation.
The Skin and Tissue Bank of the Instituto Nacional de Rehabilitación as a prototype model for the recuperation of tissuesSince March 2009, the Skin and Tissue Bank of the Instituto Nacional de Rehabilitación has been the Ministry of Health's leading tissue bank in tissue storage, within the framework of National Health Institutes. Its mission is to be a centre of excellence in the procurement, processing and storage of skin and other tissues, through the development of a model based on scientific research, the use of new biomedical and molecular state-of-the-art technologies and the education and training of highly specialised human resources.
In addition, the bank provides specialised surgical assistance for the obtention of tissues in procurement hospitals or those generating procurement, as well as their use in allogenic tissue implantation programmes. At the same time it develops donor culture awareness and outreach campaigns.
The bank reiterates the directives and principles established by the Centro Nacional de Trasplantes with the introduction of 5 essential ethical principles: voluntary donation, altruism, solidarity, confidentiality and information.
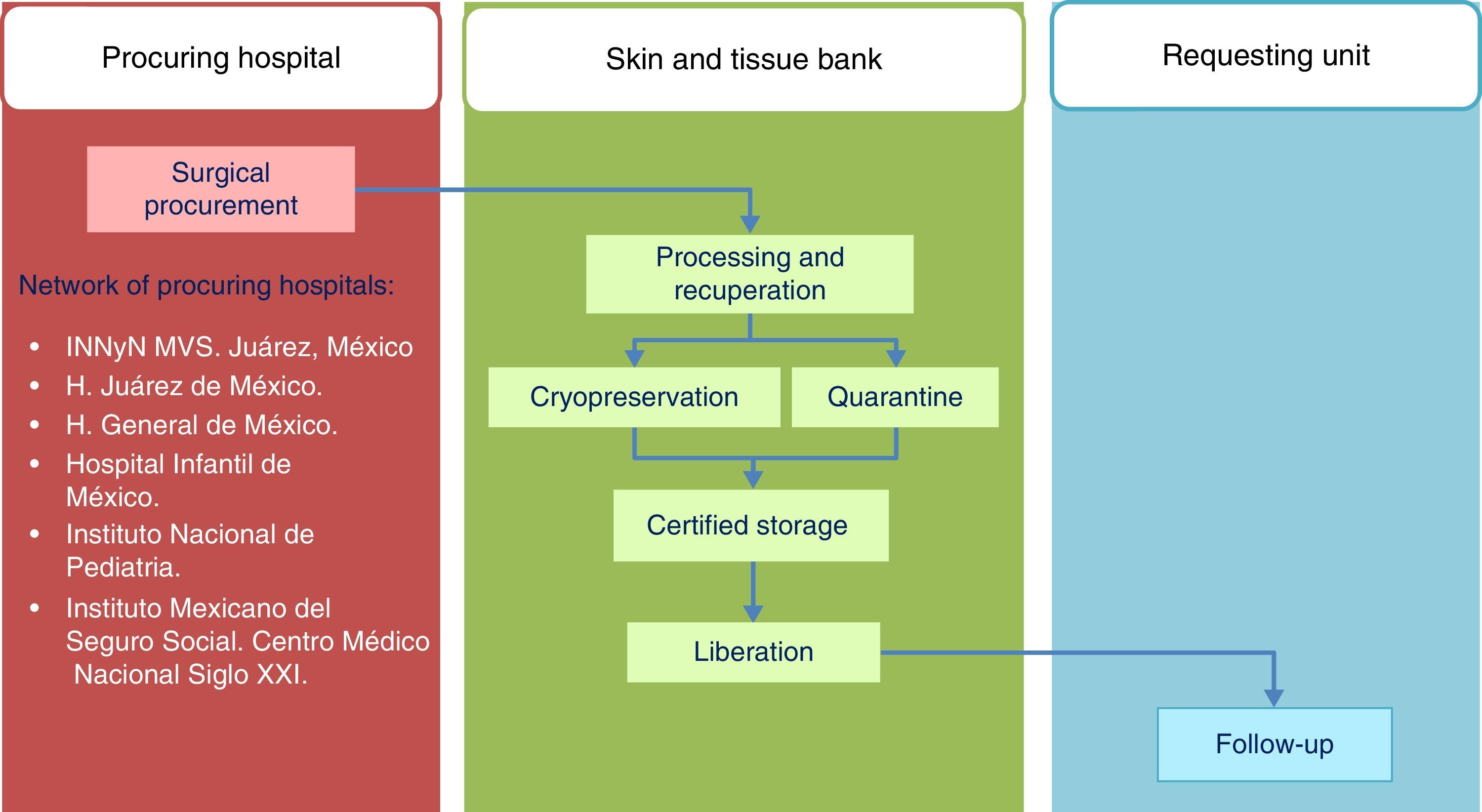
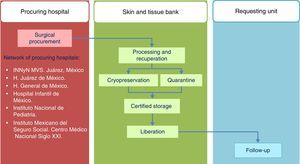
Standards of the Skin and Tissue Bank for the harvesting of skin and tissue allografts for use in transplantsThe model followed by the Skin and Tissue Bank of the and Instituto Nacional de Rehabilitación is based on complete integration of the phases involved in the tissue donation and transplant system, with traceability outlined for the procedures in line with ISO-9000-2008 (Fig. 1) regulation.
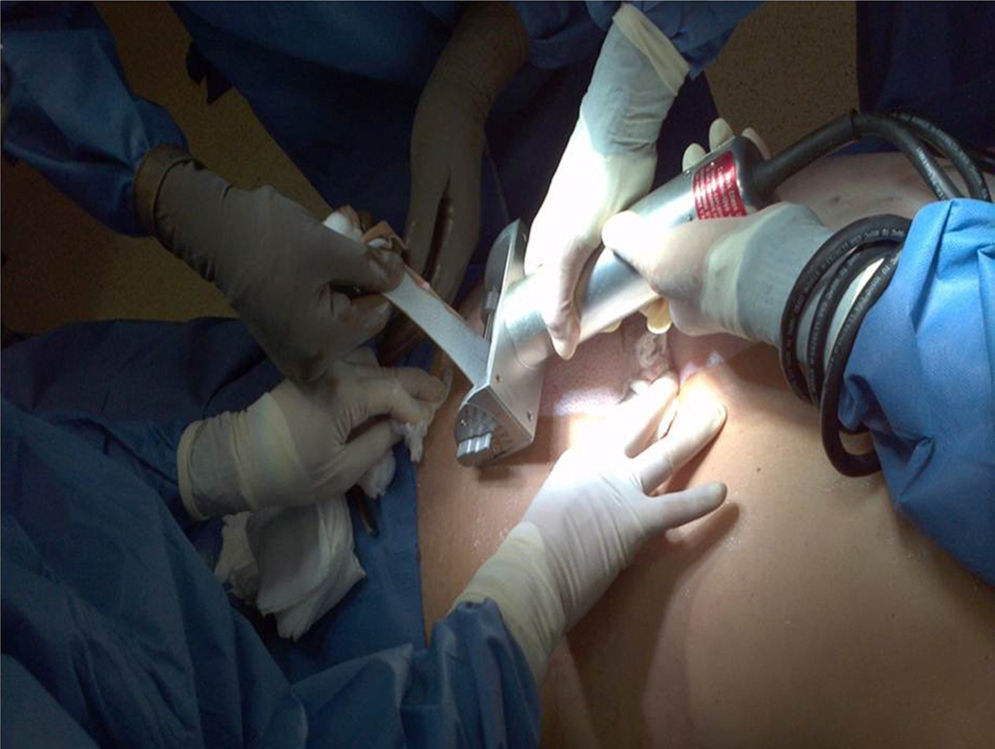
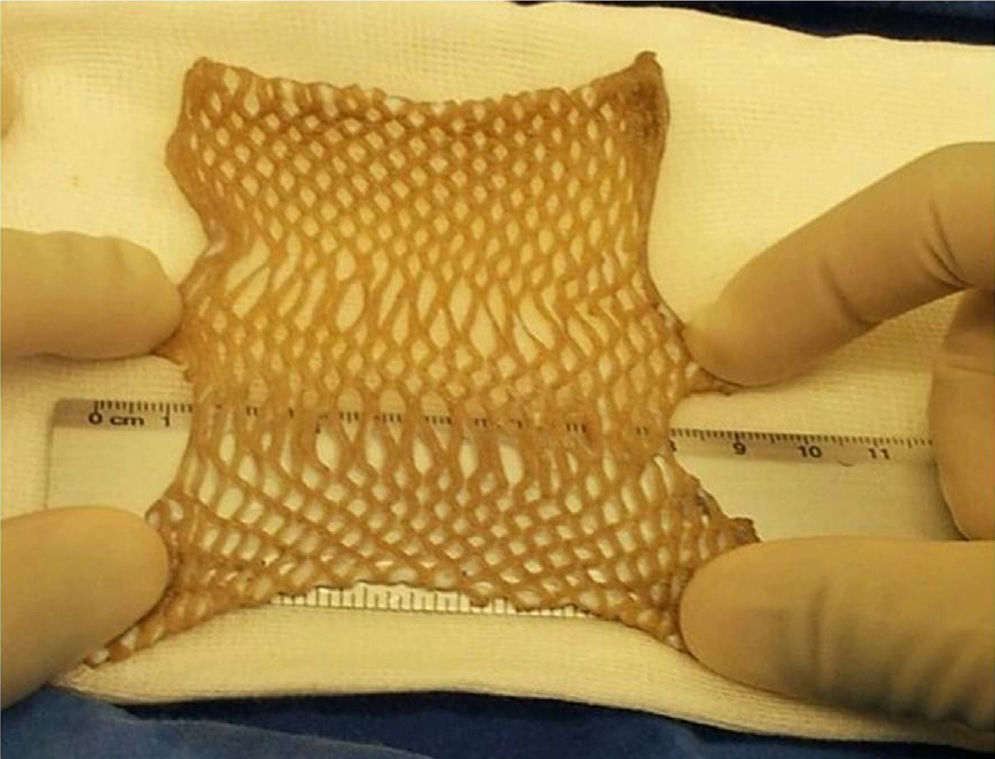
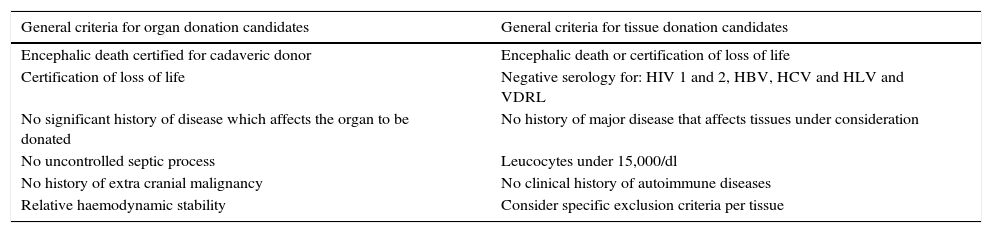
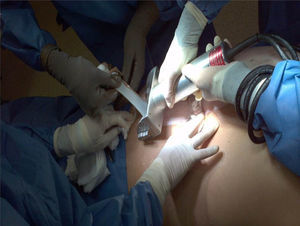
HarvestingThe procedure for obtaining skin and tissues is made through inter-institutional collaboration with different transplant programmes which meet the criteria established for organ and tissue donation (Table 2). Tissue harvesting is currently defined as a surgical event (Fig. 2) and as such quality standards for skin recovery surgery must therefore be adhered to.18
Inclusion and exclusion criteria for organ and tissue donation.
| General criteria for organ donation candidates | General criteria for tissue donation candidates |
|---|---|
| Encephalic death certified for cadaveric donor | Encephalic death or certification of loss of life |
| Certification of loss of life | Negative serology for: HIV 1 and 2, HBV, HCV and HLV and VDRL |
| No significant history of disease which affects the organ to be donated | No history of major disease that affects tissues under consideration |
| No uncontrolled septic process | Leucocytes under 15,000/dl |
| No history of extra cranial malignancy | No clinical history of autoimmune diseases |
| Relative haemodynamic stability | Consider specific exclusion criteria per tissue |
VDRL, serological test for syphilis; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLV, human lymphotropic virus.
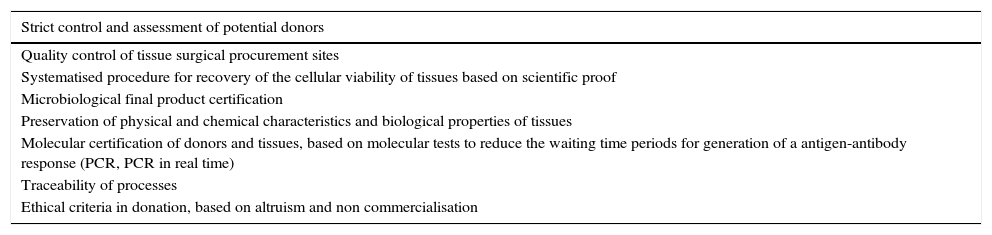
Once informed consent has been obtained from the multiple organ donor's family and cardiac arrest meets with inclusion criteria (Table 3), in the specific case of skin surface area disinfection processes are carried out based on iodine and alcohol solutions in different concentrations (70–100%), in a surgical area with an aseptic technique.
Essential directives for the recovery of tissues.
| Strict control and assessment of potential donors |
|---|
| Quality control of tissue surgical procurement sites |
| Systematised procedure for recovery of the cellular viability of tissues based on scientific proof |
| Microbiological final product certification |
| Preservation of physical and chemical characteristics and biological properties of tissues |
| Molecular certification of donors and tissues, based on molecular tests to reduce the waiting time periods for generation of a antigen-antibody response (PCR, PCR in real time) |
| Traceability of processes |
| Ethical criteria in donation, based on altruism and non commercialisation |
PCR, polymerase chain reaction.
The concept of recovery focuses on reversing or as much slowing down as possible of cell death, induced by tissue hypoxy and the depletion of nutrients, after the last heartbeat. To do this the tissues are stored in solutions formulated with a physiological pH (7.4) and with buffering agents in the presence of essential nutrients and antibiotics to begin the process of microbial decontamination. Medium term preservation is achieved using cryopreserving solutions and pH stabilisers which enable the tissue to be maintained at a temperature below −80°C. The formulation of these materials is carried out in the preparation laboratory of the tissue and skin bank itself, in keeping with the good manufacturing practice and good laboratory practice, and are sterilised by filtration.
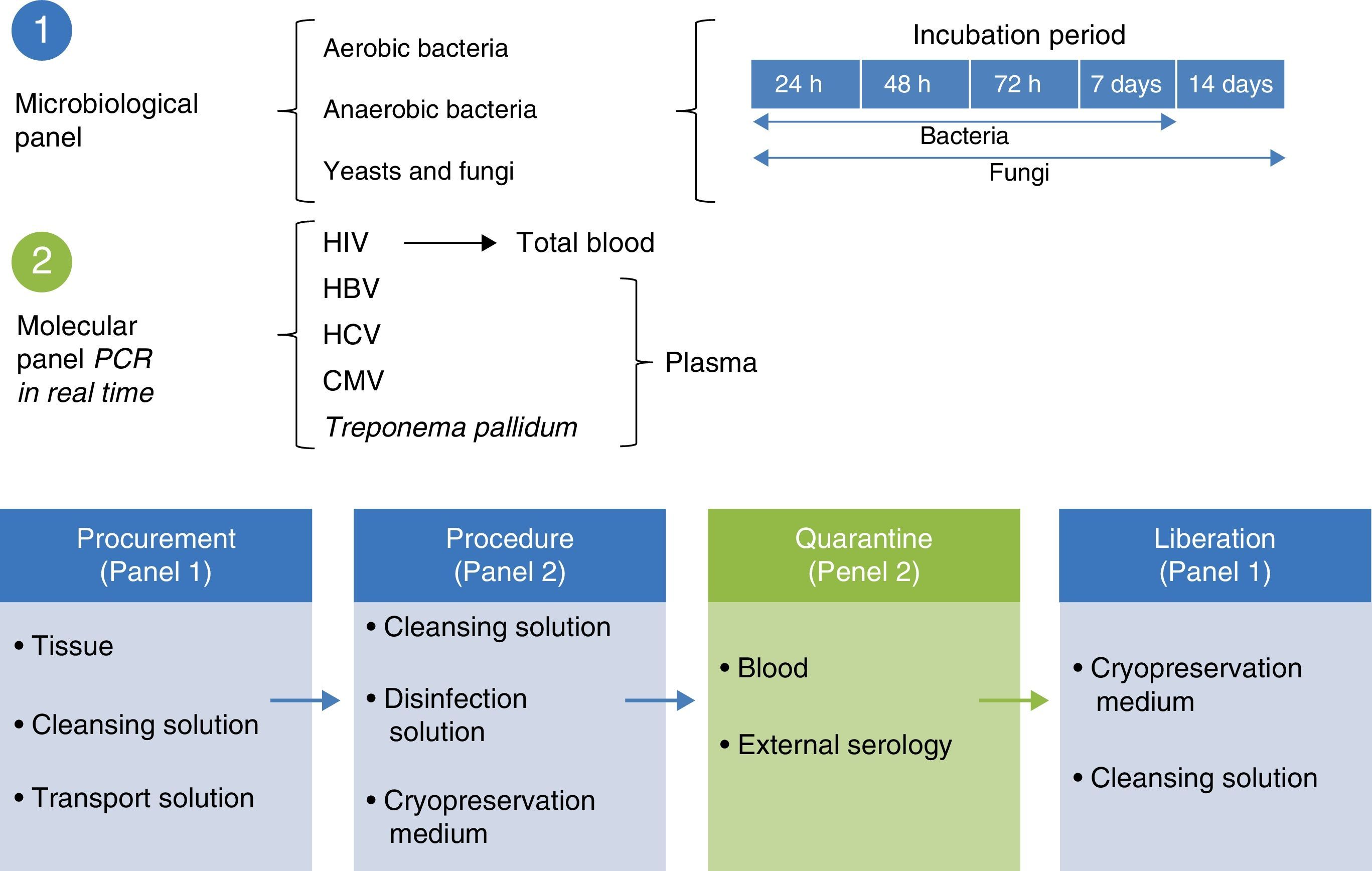
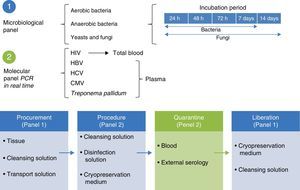
Microbiological quality certificationThe biological quality of the tissues and the microbiological certification are essential standards for tissue preservation. The standard for aerobic, anaerobic and fungal microorganisms identification is to take readings at 7, 14 and 21 days under specific mediums.19 The microbiological control system is used in tissue quality control protocols, with the use of solution to maintain contact with the recuperation and preservation process (Fig. 3).
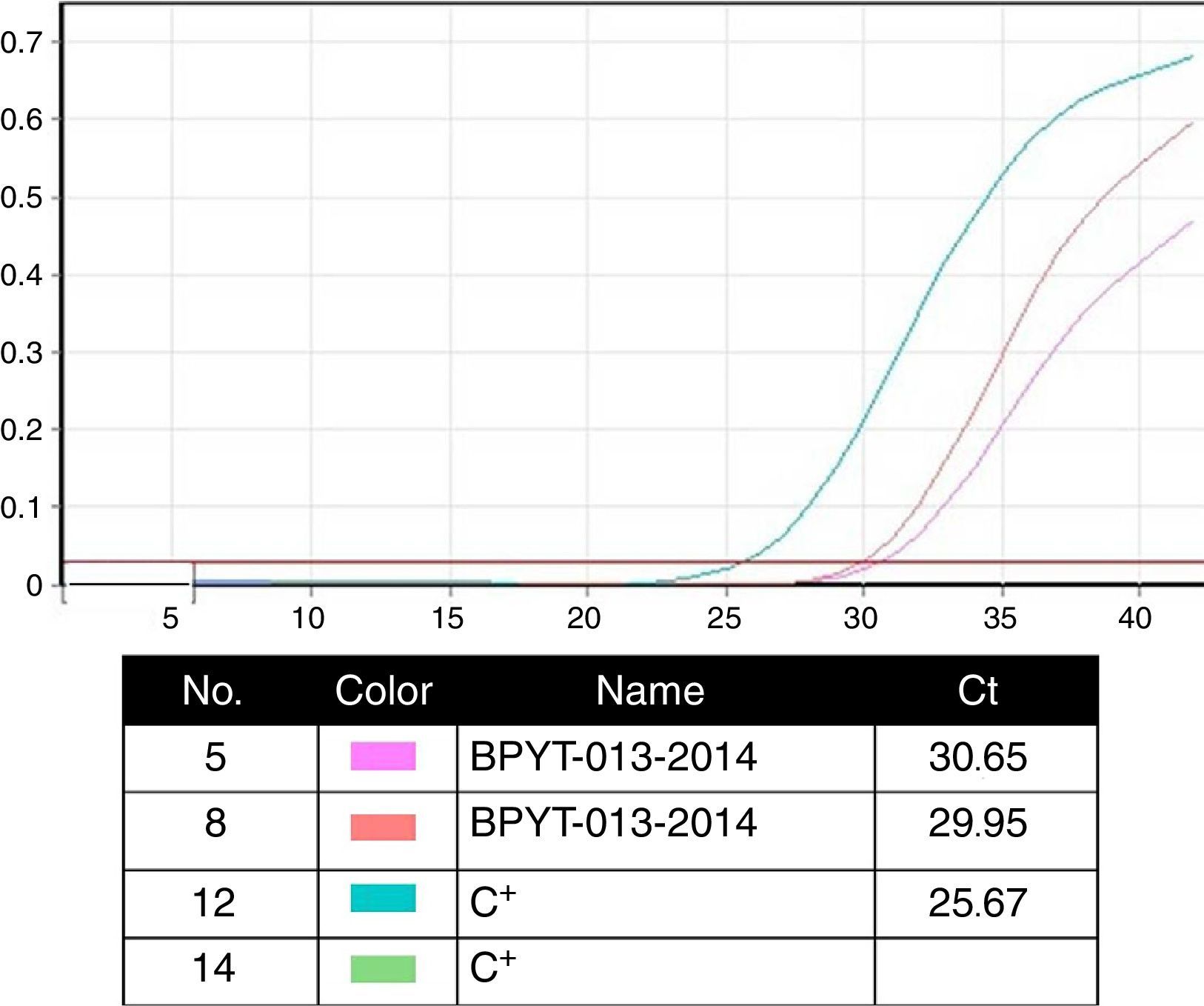
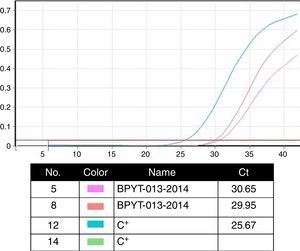
Molecular biology techniques for identification of pathogen agent identificationThe gold standard in identifying pathogens is carried out with the identification of genomic segments using the polymerase chain reaction technique (PCR). This technique which was described by Kubista Mullis and his team20 has a sensitivity rate of 1×10−7; i.e. it is able to identify an infected cell for every 1×107 healthy cells. In the case of certification protocols, the tests which the banks perform are made in real time, searching for the human immunodeficiency virus types 1 and 2, hepatitis B and C; cytomegalovirus and Treponema pallidum. Given that the technique is highly specific and sensitive, tests are made in triplicate in a sterilised area and safety cabinet with laminar flow type II-AB. These tests are validated for diagnostic and clinical use in humans and include internal controls (calibrated as negative and positive) for each test with a search in donor blood and tissue (Fig. 4).6,20
Physical infrastructureAt present the bank consists of 5 laboratories, which meet with class 100 regulations and operate according to good manufacturing practice and good laboratory practice. Since this is a national reference centre, the bank used its physical infrastructure meeting with directives from the Federación Española de Bancos de Tejidos and the Asociación Europea de Bancos de Tejidos. It is physically structured according to 3 main areas10: (1) class 100 inner chamber; (2) administrative area; and (3) support and research areas.
Class 100 inner chamber structureLaboratory for the preparation of materialsEquipment and materials for the preparation of cleansing, decontamination and maintenance solutions at constant temperature (18°C), filtered air (class 100) and positive pressure.
Quarantine laboratoryClean room with constant temperature (18°C), filtered air (class 100) and positive pressure for recovery and decontamination of tissues. Refrigerators for maintaining a constant temperature (4°C) in decontamination processes. Class IIAB biological safety cabinet for handling tissue.
Processing laboratory for musculoskeletal tissueDouble-chamber clean room insulated with a constant temperature, filtered air (class 100) and positive pressure aimed at the recovery, cutting and decontamination of musculoskeletal tissue.
Packaging laboratoryClean room with constant temperature (18°C), filtered air (class 100) and positive pressure, for final tissue packaging prior to being sent for final freezing. The processes are carried out in a class IIA-B biological safety cabinet for tissue handing. The inner chamber connects with an area for tissue storage and safekeeping.
Tissue storage and safekeeping area (deep freezing)Restricted access area for tissue storage with temperature support systems, monitoring system in real time for temperature recording and a televised surveillance system. Constant temperature (18°C) and class 100 filtered air.
Equipment and Sterilisation Centre (CEYE)Equipment and supplies for validating sterilisation processes of materials and equipment used in procurement processes.
Administrative areaDesigned outside central facilities and with restricted access.
Support and research areasConstituted by a laboratory of multiple uses and with a culture chamber, for the development of research protocols relating to the formulation of materials, cellular death blockage mechanisms and cryobiotic molecular strategies for the purpose of transplants.
Discussion and perspectivesIn keeping with the worldwide trend to recuperate tissues for therapeutic uses, the storage of skin and other tissues in the context of healthcare needs and current Mexican medical research several of the following points must be precisely defined to obtain a better therapeutic outcome:
Conceptualise the procurement and harvesting of tissues as a surgical eventThis point necessitates a change in the conceptualisation of the obtainment of tissues as a surgical event, with particular emphasis on quality, as recently described in detail in the lasts amendment to the Ley General de Salud, relating to the regulation governing organs and tissues (12th December 2011).
Defining the type of procedure used in preservationEach method (chemical decontamination, lyophilisation, gamma sterilisation) has a different effect on the biological, physical and chemical characteristics of the tissue (cellular viability index, loss of water, of elasticity and resistance), and finally, in its posterior usage. In our model the preliminary data indicate that the biological recuperation of tissues represents the method with the best therapeutic outcome, following a long period of freezing at temperatures below 80°C below zero (Fig. 5).
Traceability of processesDocumentation of each phase of procurement, preservation and liberation of tissue, respecting privacy and confidentiality data.
The traceability of processes must document the critical route of each process and include essential documents such as: (1) manual for specific procurement procedure; (2) manual for specific procedure for recuperation and preservation of tissues; (3) manual for microbiological control and specific tissue quality; (4) procurement protocol for tissue procuring hospitals; (5) clinical files and service formats (microbiological, molecular laboratory, pathology, etc.); (6) manual and records of preventative maintenance of equipment and installations; (7) registry and control of access for general services and microbiological records of environmental controls; and (8) registry and report of activities sent to the Centro Nacional de Trasplantes.
In addition, the following lines of action may have a serious impact on the growth of tissue bank activity:
Integration and growth of a hospital network for increasing regional tissue affluenceEstablishing a network for the procurement of tissues involves carrying out a large number of collaboration agreements in tissue procurement matters, for transplant in other hospital institutions, for the purpose of increasing the identification of potential tissue donors.
Implementation of a research programme in tissue pharmaceutical cryobiotics with therapeutic usesThe aim of this is to promote the design of procurement and cryopreservation protocols for skin and tissues, and also to foster the broadening of scientific research to reduce dependency on imported materials and promote self-sustainability of operations, focused on: (1) scientific procedures for preservation and tissue cryobiology aimed at obtaining high quality biological and therapeutically effective products; (2) generation of materials and patents for the bank's self sustainability; and (3) scientific dissemination of processes used in other institutions.
Implementation of an academic surgical training programme for tissue procuratorsArranging the registration and execution of multi-tissue procurator courses, endorsed by both academic institutions and the Centro Nacional de Trasplantes In Mexico to form specialised and highly trained personnel. Furthermore, to include specific modules on the surgical procedures involved in tissue procurement and transplant in pre-grade modules study programmes.
Advocacy of a more widespread cadaveric skin donation cultureDriving the training programme for leaders who will be the promoters of the culture of organ and tissue donation in collaboration with non-governmental organisations and mass media communication campaigns, with use of social networks and internet.
Last but not least, several ethical considerations have to be readdressed, starting with the fact that the obtainment of tissues is an act of altruistic donation with no profit making or commercialisation and process recuperation quotas must therefore be regulated and defined; this point is also being addressed by the World Health Organisation.
The coordination and integration of the organ and tissue donation and transplant programmes must be carried out with functional, responsible and equitable participation from areas of research, human resources training and health care services.
This model is merely one of the medium term proposals to modify the current tissue recovery setting. Possibly in a couple of decades, similarly to the Spanish model it could become the world leader of organ and tissue donor programmes.
FinancingThis study was financed by the Council for Science and Technology through the Fondos Sectoriales de Investigación in Salud CONACYT-FOSIS 2011-1-161624.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Francisco Martínez-Flores F, Sandoval-Zamora H, Machuca-Rodriguez C, Barrera-López A, García-Cavazos R, Madinaveitia-Villanueva JA. Banco de piel y tejidos: un modelo operativo para la recuperación y preservación de aloinjertos de piel y tejidos. Cir Cir. 2016;84:85–92.