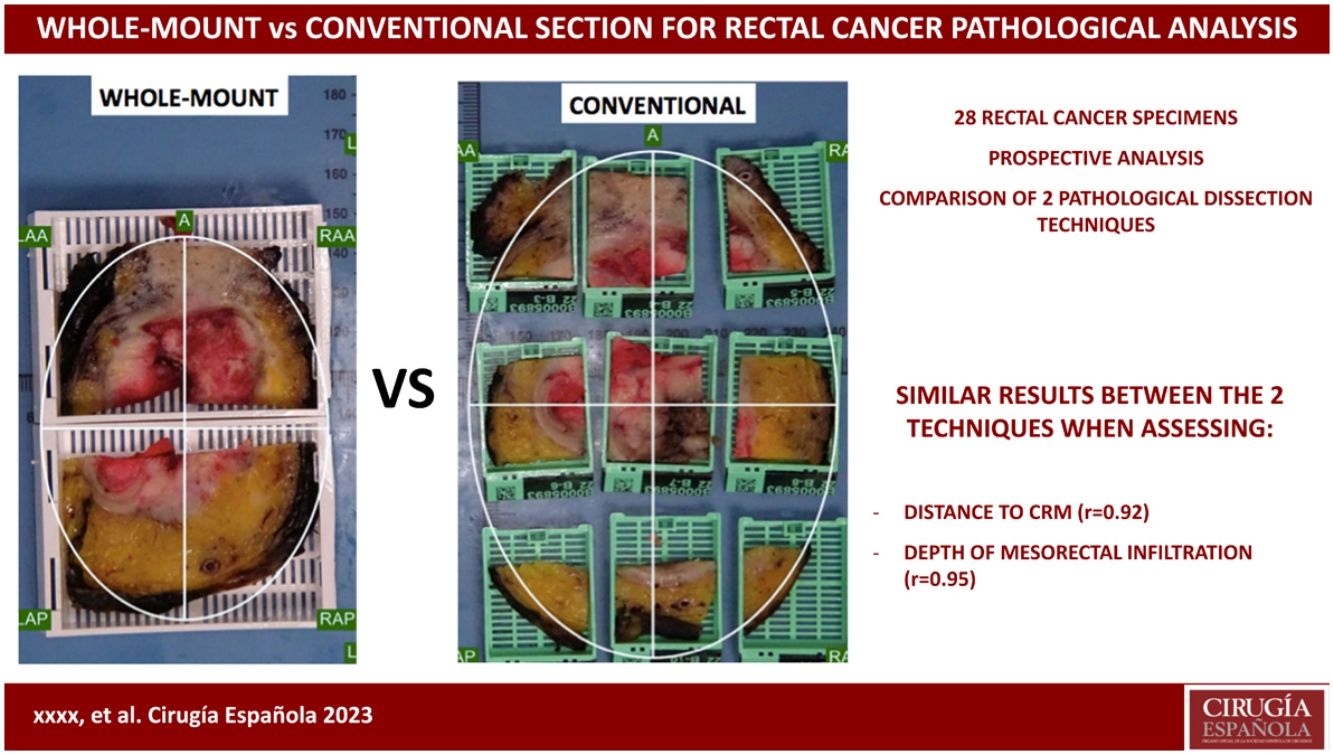
The objective of the study is to compare 2 techniques for histological handling of rectal cancer specimens, namely whole-mount in a large block vs conventional sampling using small blocks, for mesorectal pathological assessment of circumferential resection margin status and depth of tumor invasion into the mesorectal fat.
MethodsThis is a prospective study including 27 total mesorectal excision specimens of rectal cancer from patients treated for primary rectal carcinoma between 2020 and 2022 in a specialized multidisciplinary Colorectal Unit. For each total mesorectal excision specimen, 2 contiguous representative tumoral slices were selected and comparatively analyzed with whole-mount and small blocks macroscopic dissection techniques, enabling comparison between them in the same surgical specimen. The agreement between the 2 techniques to assess the distance of the tumor from the circumferential resection margin as well as the depth of tumor invasion was evaluated with the Student’s t-test for paired samples, Pearson’s correlation coefficient, and the Bland-Altman method comparison analysis.
ResultsComplete mesorectal excision was observed in 8% of cases. Circumferential resection margin involvement was observed in only one case (4 %). The whole-mount and small block techniques obtained similar results when we assessed the distance to the circumferential resection margin (t-test P = 0.8, r = 0.92) and the depth of mesorectal infiltration (t-test P = 0.6, r = 0.95).
ConclusionsBoth gross dissection techniques (whole-mount vs multiple small cassettes) are equivalent and reliable to assess the distance to circumferential resection margin and the depth of mesorectal infiltration in the mesorectal fat in rectal cancer staging.
El objetivo del estudio es comparar dos técnicas para el manejo histológico de especímenes de cáncer de recto (el montaje completo en un bloque grande frente al muestreo convencional utilizando bloques pequeños) para la evaluación patológica del estado del margen de resección circunferencial y la profundidad de infiltración tumoral en la grasa mesorrectal.
MétodosEste es un estudio prospectivo que incluyó veintisiete especímenes de escisión total de mesorrecto de pacientes tratados por carcinoma de recto primario entre 2020 y 2022 en una Unidad Colorrectal multidisciplinaria especializada. Para cada especimen se seleccionaron dos cortes tumorales representativos contiguos que fueron analizados de manera comparativa utilizando las técnicas de disección macroscópica de montaje completo y la de bloques pequeños, permitiendo una comparación entre ellas en el mismo espécimen quirúrgico. La concordancia entre las dos técnicas para la evaluación de la distancia del tumor al margen de resección circunferencial y la profundidad de infiltración tumoral se evaluó con la prueba t de Student para muestras pareadas, el coeficiente de correlación de Pearson y el análisis de comparación de métodos, descrito por Bland y Altman.
ResultadosSe observó una escición completa del mesorrecto en el 85%de los casos. El margen de resección circunferencial se consideró afecto en solo un caso (4%). Las técnicas de montaje completo y bloques pequeños obtuvieron resultados similares al evaluar la distancia al margen de resección circunferencial (p = 0,8 en la prueba t, r = 0,92) y la profundidad de infiltración mesorrectal (p = 0,6 en la prueba t, r = 0,95).
ConclusionesAmbas técnicas de disección macroscópica (montaje completo vs. múltiples bloques pequeños) son equivalentes y fiables para evaluar la distancia al margen de resección circunferencial y la profundidad de infiltración tumoral en la grasa mesorrectal en la estadificación del cáncer de recto.
The circumferential resection margin (CRM) and the quality of the mesorectum after total mesorectal excision (TME) are 2 crucial pathological factors in rectal cancer treatment.1–5 Moreover, the microscopic assessment of the depth of mesorectal invasion (DMI) beyond the outer border of the muscular layer helps to subclassify pT3 tumors, as a DMI ≥ 5 mm has been associated with significantly worse survival outcomes.6,7
CRM status is an independent prognostic factor of local recurrence and poor survival after rectal cancer surgery, and it has been used as an indicator of the quality of surgery in several national registries.8–12 However, the methodology to assess CRM involvement in rectal carcinoma varies significantly among the different published articles.1,13–15 Moreover, CRM involvement rates show a wide variation between different studies.16 The percentage of CRM-positive patients depends on patient selection, performance of preoperative imaging, use of preoperative long-term therapy, surgical technique, and skill of the pathologist. In part, it also could be due to the different pathological methods applied to assess it. Thus, more careful examination of the CRM would improve prediction of local recurrence and allow for standardization of pathological reporting, which would be beneficial for interpretation of multicenter trials.16
Quirke et al.,1 in their original article about pathological rectal cancer evaluation, described the use of whole-mount (WM) sections of the mesorectum to assess both CRM and DMI. WM histopathology refers to the histopathological examination of tissue sections from specimens processed with large tissue cassettes (mega-cassette), where the whole slice may be studied, instead of traditional histology based on regular cassettes. Implementation of large-format histology started in the 1970s with brain pathology, and currently it is still routinely applied in specimens of breast and genitourinary cancer at some instutitions.17,18
Although the vast majority of CRM studies on rectal cancer have quoted the original description by Quirke1 in 1986, the WM section technique is specifically reported only by a few authors in clinical practice,19,20 in anatomical studies on morphometry, and reports on surgical pitfalls in TME and MRI accuracy in preoperative assessment.21–23 Many other authors do not specify whether the WM technique is used for CRM status assessment,8,24,25 while others specifically mention not using it at all.26 Nowadays, many pathologists probably do not use mega-cassettes to include whole-mount slices, because microtomes with mega-cassette clamps are not easily available in all labs and the procedure is technically tricky because of the amount of adipose tissue.
The absence of standardized pathology processing and reporting might limit the validity of the conclusions of some papers on CRM. The purpose of this study was to compare the accuracy of 2 techniques – WM in a large block (mega-cassette) vs conventional gross sampling using small blocks (SB) – for mesorectal pathological assessment of CRM status and DMI.
MethodsThis is a prospective study including twenty-eight specimens of TME from non-consecutive patients treated for primary rectal carcinoma with curative intention between November 2020 and July 2022 in a specialized Multidisciplinary Colorectal Unit. The study was approved by the local institutional review board and all the patients signed an informed consent to participate in the study. Data are reported according to the STROBE guidelines.27
For each TME specimen two contiguous representative tumoral slices were selected to analyze and compare CRM and DMI measurements obtained from two different gross dissection techniques: WM using mega-cassettes vs conventional sampling using SB or standard cassettes.
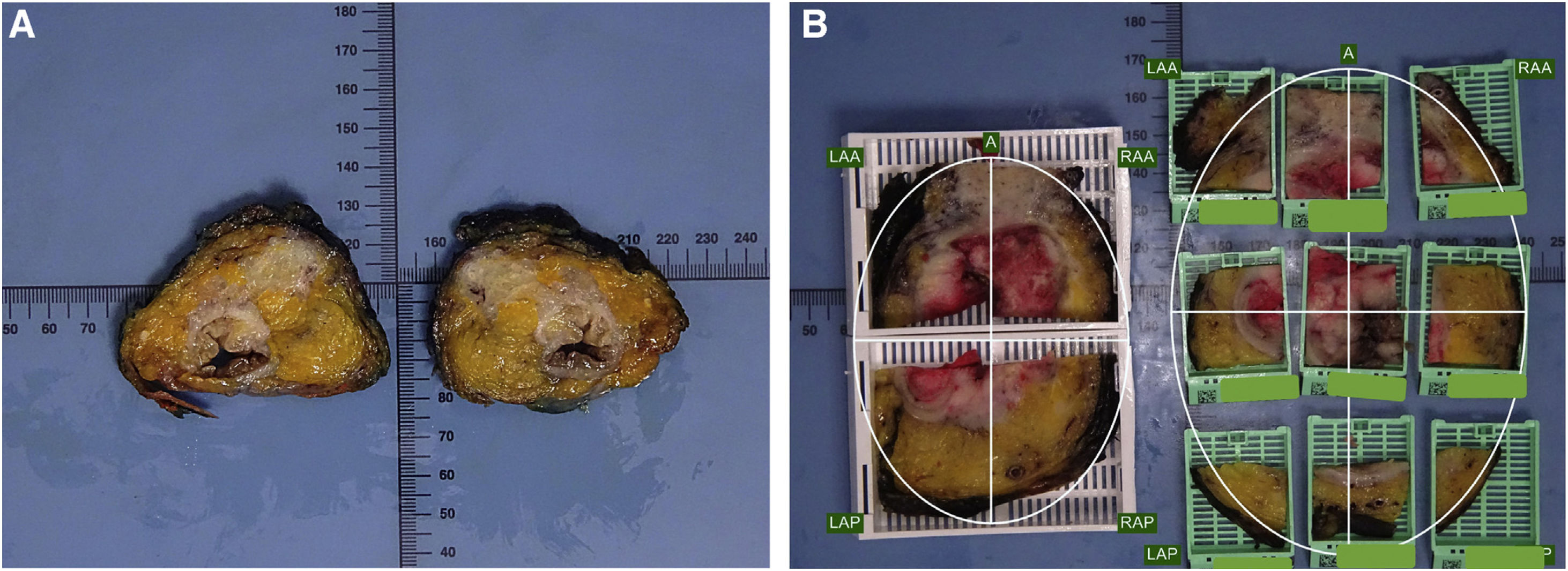
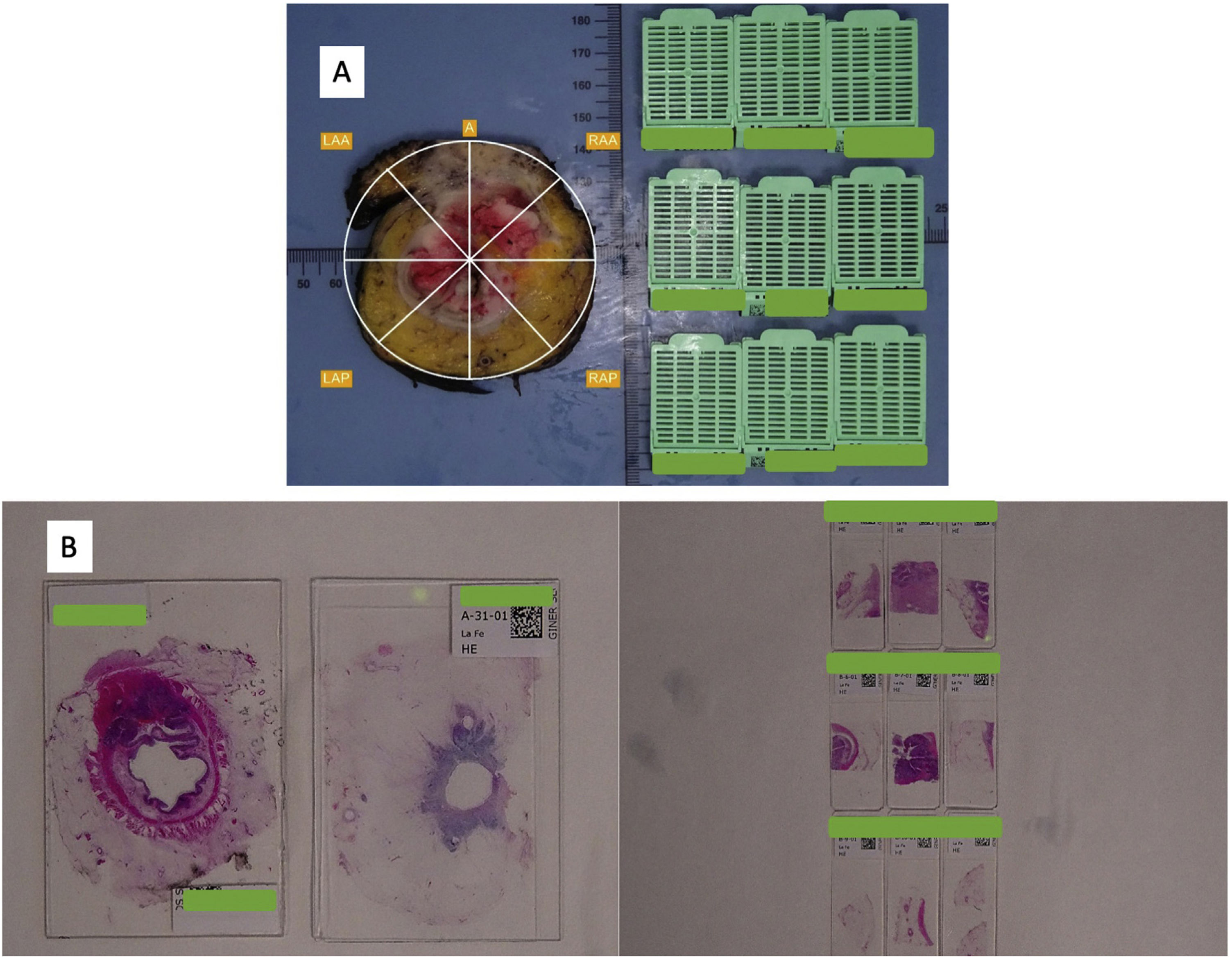
Pathologic dissection of each specimen was carried out by the same pathologist (FG) in a standardized fashion in accordance with the technique described by Quirke et al.1,2,28 The resected specimen was thoroughly examined with the naked-eye to assess the quality of mesorectal excision, marked with ink on its extraperitoneal surface, and fixed in 4% formalin for 48 h. Thereafter, transverse slices of the tumor were made, and the segments above and below were examined at approximately 3- to 5-mm intervals, looking for continuous spread of tumor up to the CRM, or the presence of discontinuous tumor deposits or lymph node involvement at the CRM. As judged grossly, we selected the most infiltrative slice to be included in a mega-cassette for WM study, and the immediately adjacent slice was divided into sections to be included in standard (conventional) cassettes or SB (Figs. 1 and 2). The directly opposing surfaces of the 2 slices were examined (ie, the front of one slice, and the back of the opposing one) (Fig. 1a). The conventional sections for SB were oriented and organized in a clock-face manner (proceeding clockwise, specified as anterior, posterior, right/left lateral, or in squares (Figs. 1 and 2).
(A) Two “mirror” sections are selected to be included by both techniques (WM and SB) in a specimen with neoadjuvant treatment; (B) The comparison of both inclusion techniques (WM and SB) of 2 adjacent slices; A: anterior; LAA: left anterior; RAA: right anterior; LAP: left posterior; RAP: right posterior.
All blocks (standard and mega-cassettes) were embedded in paraffin wax, sectioned and stained with hematoxylin and eosin (H&E) (Fig. 2B). The sections were examined microscopically, using an optic microscope (Leica®, DMD 108) and the Vernier scale for CRM and DMI measurements in mm to compare both techniques. Other pathological parameters were also assessed, including differentiation grade, T and N stages, lymphovascular and perineural invasion, and regression grade in case of neoadjuvant therapy.
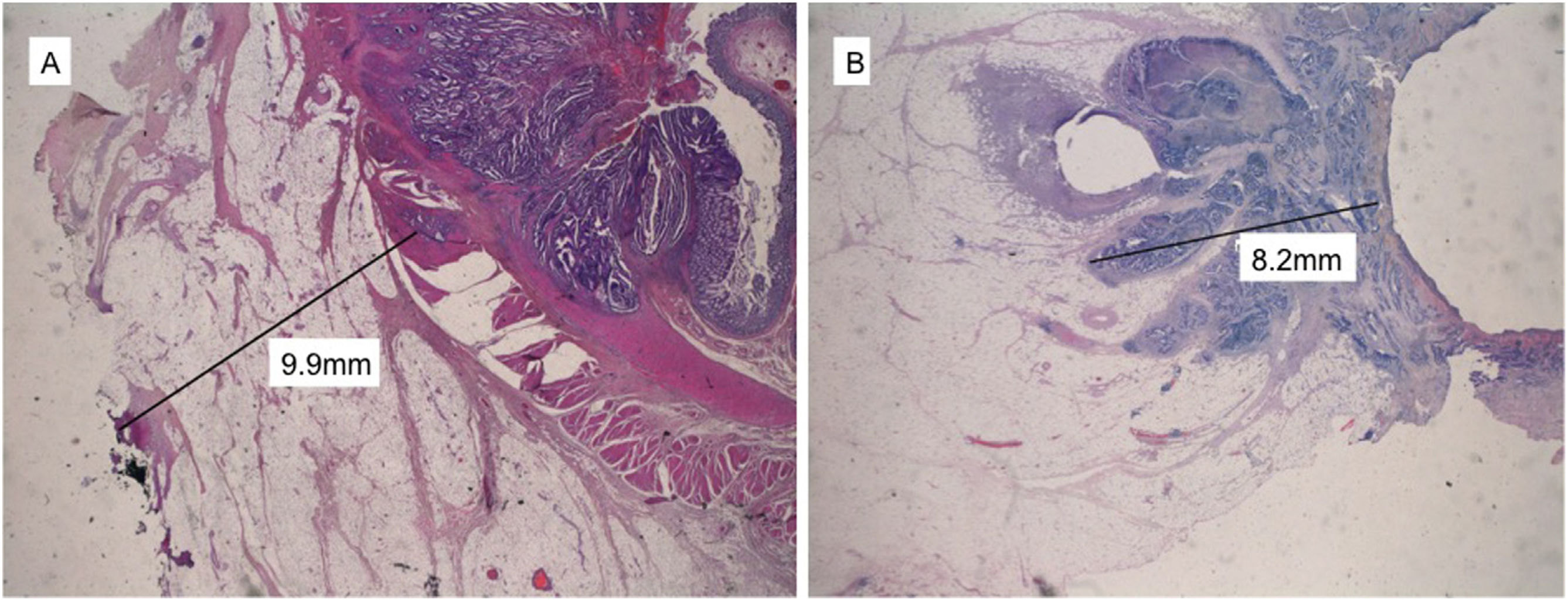
Distance to CRM (dCRM) was defined as the shortest distance between the tumor and the resection plane (Fig. 3A). CRM involvement was determined by the presence of microscopic tumor cells either on the inked surface or at a distance ≤1 mm from the margin for direct tumor spread.16
Using the same procedure, DMI was also measured comparatively by the WM and SB techniques (Fig. 3B). DMI was measured in mm from the outer edge of the muscle layer to the maximum outer margin of the tumor. In cases in which the muscle layer was completely disrupted by the tumor, DMI was measured from the intraluminal tumor surface (Fig. 3B).
Statistical analysisThe variables were described as number of patients and percentage for categorical variables, or mean, median and range for continuous variables.
Agreement between the 2 techniques (WM vs SB) in the evaluation of the outcome variables (dCRM and DMI) was firstly assessed by comparing the mean values with the Student’s t-test for paired samples. Then, the values obtained with both techniques were compared using a Pearson correlation coefficient and reported in a scatter plot. Finally, agreement between them was assessed using the method comparison analysis described by Bland and Altman,29 and proportion bias was evaluated performing a linear regression including the difference and the mean value.
The Statistical Package for the Social Sciences (SPSS version 22.0, USA) was used for the data management and statistical analyses. P values <.05 were considered statistically significant.
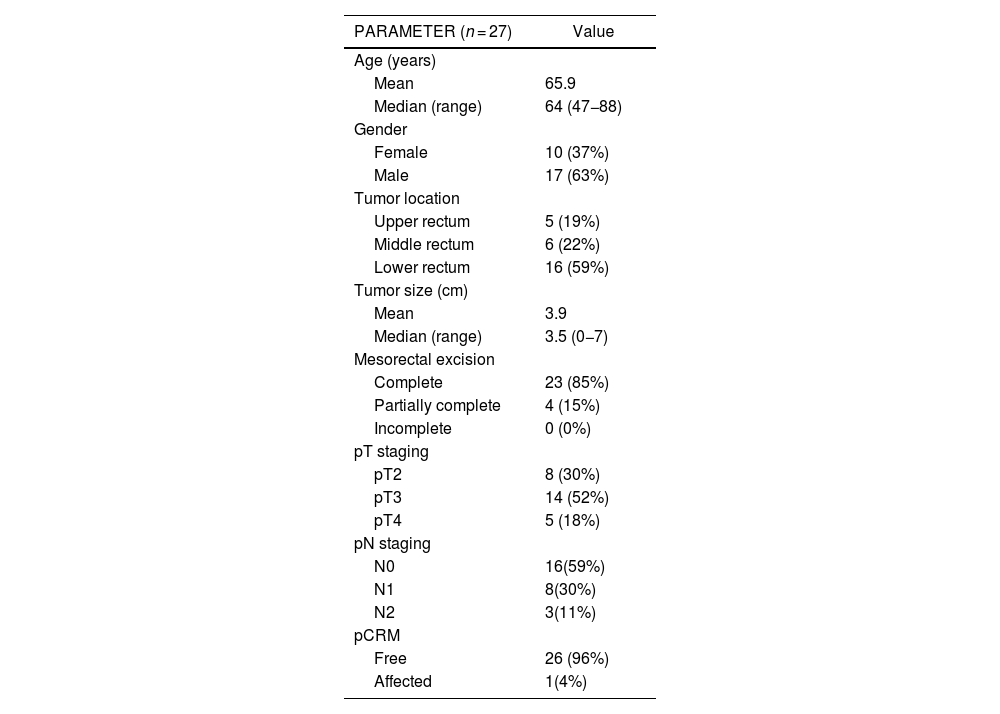
ResultsAmong the 28 patients initially enrolled in the study, one was excluded due to achieving a pathological complete response ypT0N0 after neoadjuvant treatment, resulting in the inclusion of 27 patients for the final analysis. All demographic and clinicopathological data are reported in Table 1. Nine cases received preoperative chemo-radiotherapy, with different grades of response. Considering the Dworak’s classification, we observed 2, 3 and 3 cases with grades 1, 2 and 3 of response to neoadjuvant therapy, respectively. Eleven cases showed lymph node involvement (N+) (Table 1). pCRM showed involvement in only one patient (4%).
Demographic and clinicopathological characteristics of patients.
| PARAMETER (n = 27) | Value |
|---|---|
| Age (years) | |
| Mean | 65.9 |
| Median (range) | 64 (47−88) |
| Gender | |
| Female | 10 (37%) |
| Male | 17 (63%) |
| Tumor location | |
| Upper rectum | 5 (19%) |
| Middle rectum | 6 (22%) |
| Lower rectum | 16 (59%) |
| Tumor size (cm) | |
| Mean | 3.9 |
| Median (range) | 3.5 (0−7) |
| Mesorectal excision | |
| Complete | 23 (85%) |
| Partially complete | 4 (15%) |
| Incomplete | 0 (0%) |
| pT staging | |
| pT2 | 8 (30%) |
| pT3 | 14 (52%) |
| pT4 | 5 (18%) |
| pN staging | |
| N0 | 16(59%) |
| N1 | 8(30%) |
| N2 | 3(11%) |
| pCRM | |
| Free | 26 (96%) |
| Affected | 1(4%) |
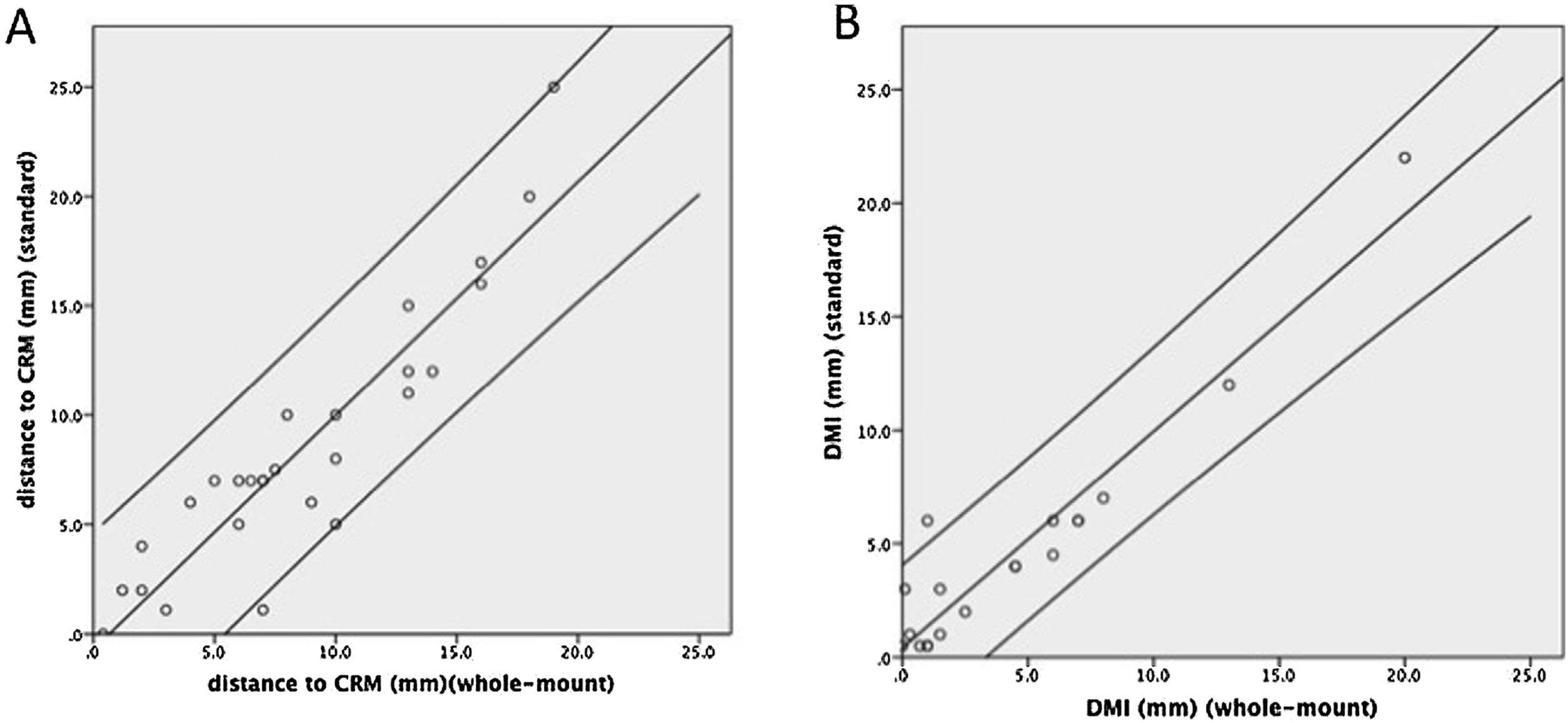
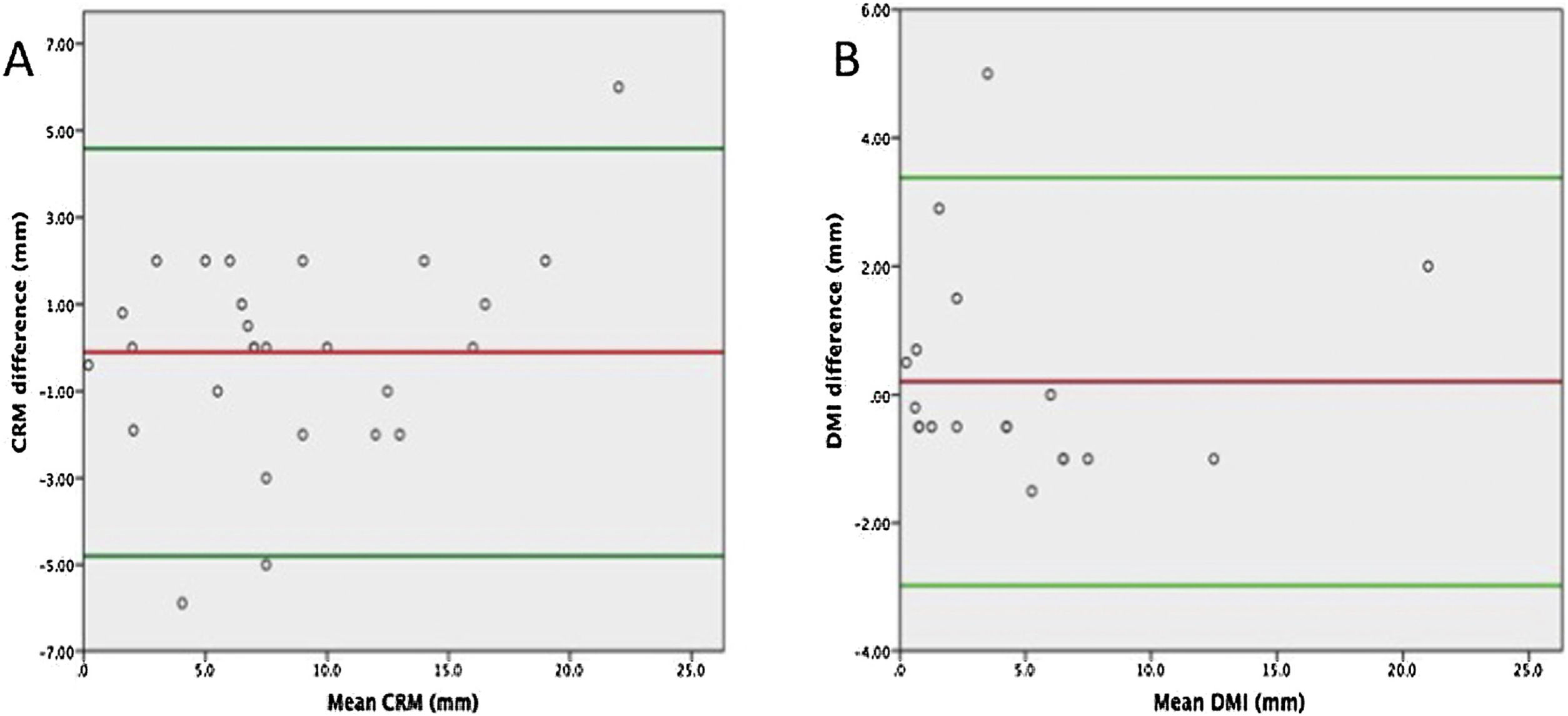
Complete staging agreement between the 2 techniques (WM vs SB) was achieved for pTN and CRM involvement in all patients. The mean distance observed to CRM was similar comparing both techniques: 8.5 (SD 6.0) mm and 8.7 (SD 5.2) mm for the SB and WM techniques, respectively (P = 0.8) showing a good correlation coefficient r = 0.92 (P < 0.001) (Fig. 4A). The Bland-Altman plot (Fig. 5A) showed that in only 3 cases was the difference between the 2 techniques out of the 95% confidence interval. Differences in the measurements made by the SB vs WM technique have a clear tendency to increase with increasing distances from the CRM, even if the regression analysis between the difference and mean was not statistically significant (P = 0.07).
Bland–Altman plot of distance to circumferential resection margin (CRM) (A) and depth of mesorectal infiltration (DMI) (B) pathologically assessed by standard vs whole-mount techniques. The red line indicates the mean difference between the 2 techniques and the green lines the 95% confidence interval.
The mean DMI was similar between the 2 techniques: 4.7 (SD 5.1) mm, and 4.5 (SD 5.1) mm for the SB and WM techniques, respectively (P = 0.6). In pT3 tumors, the mean was 4.4 mm in both techniques (range 0.5–22 mm and 0.1–20 mm by conventional and WM sections, respectively). The mean DMI in pT4 tumors was 8 mm by SB and 6.8 mm by WM dissection (range 4–20 mm and 1–15 mm by conventional and WM dissection, respectively). Correlation between the measures made by the two techniques was good, with a Pearson correlation coefficient of r = 0.95 (P < 0.001) (Fig. 4B). The Bland-Altman plot (Fig. 5B) showed that in only one case was the difference out of the 95% confidence interval, with no evidence of proportion bias (regression analysis between difference and mean: P = 0.9).
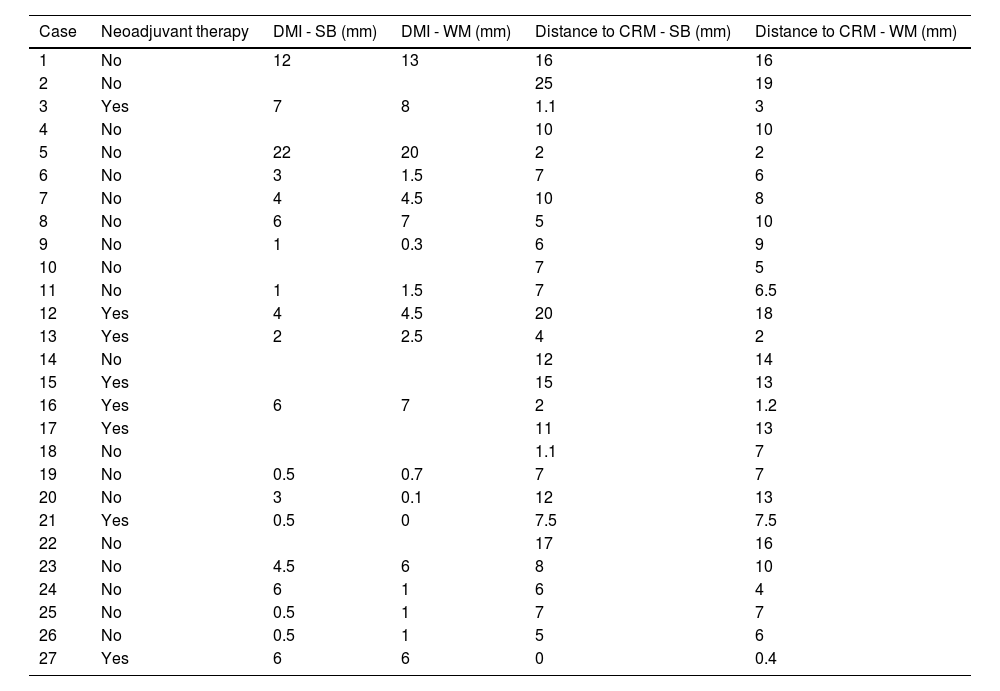
DMI and distance to CRM measured with each technique are reported in detail for each patient in Table 2.
DMI and distance to CRM measured with each technique for each patient.
| Case | Neoadjuvant therapy | DMI - SB (mm) | DMI - WM (mm) | Distance to CRM - SB (mm) | Distance to CRM - WM (mm) |
|---|---|---|---|---|---|
| 1 | No | 12 | 13 | 16 | 16 |
| 2 | No | 25 | 19 | ||
| 3 | Yes | 7 | 8 | 1.1 | 3 |
| 4 | No | 10 | 10 | ||
| 5 | No | 22 | 20 | 2 | 2 |
| 6 | No | 3 | 1.5 | 7 | 6 |
| 7 | No | 4 | 4.5 | 10 | 8 |
| 8 | No | 6 | 7 | 5 | 10 |
| 9 | No | 1 | 0.3 | 6 | 9 |
| 10 | No | 7 | 5 | ||
| 11 | No | 1 | 1.5 | 7 | 6.5 |
| 12 | Yes | 4 | 4.5 | 20 | 18 |
| 13 | Yes | 2 | 2.5 | 4 | 2 |
| 14 | No | 12 | 14 | ||
| 15 | Yes | 15 | 13 | ||
| 16 | Yes | 6 | 7 | 2 | 1.2 |
| 17 | Yes | 11 | 13 | ||
| 18 | No | 1.1 | 7 | ||
| 19 | No | 0.5 | 0.7 | 7 | 7 |
| 20 | No | 3 | 0.1 | 12 | 13 |
| 21 | Yes | 0.5 | 0 | 7.5 | 7.5 |
| 22 | No | 17 | 16 | ||
| 23 | No | 4.5 | 6 | 8 | 10 |
| 24 | No | 6 | 1 | 6 | 4 |
| 25 | No | 0.5 | 1 | 7 | 7 |
| 26 | No | 0.5 | 1 | 5 | 6 |
| 27 | Yes | 6 | 6 | 0 | 0.4 |
DMI: depth of mesorectal invasion. SB: small-block technique, WM: whole-mount technique. CRM: circumferential resection margin.
Although some studies30,31 have suggested that WM sections provide a more accurate and effective assessment of rectal cancer, especially in the spread in the mesorectal region of discontinuous microscopic tumor nodules compared to conventional sections, this study shows that both WM and the SB conventional techniques in a clockwise manner are equivalent in terms of histopathological accuracy for assessing CRM status and DMI. To our knowledge, the present study is the first comparative study of both methods.
Accurate information about CRM status is crucial for current management of rectal cancer due to its prognostic relevance for local recurrence, metastasis and survival.16 Its usefulness and relevance has been evident in national TME registries4,9,12,24,25,32 and in comparative trials on conventional vs laparoscopic TME surgery33,34 as well as transanal TME registries.35
Pathologic assessment of DMI is also an important prognostic factor in pT3 stages.14 The degree of extramural spread was a statistically significant prognostic factor for survival and local recurrence. Several studies have shown that the subdivision of category T3 rectal cancer into 2 subgroups of extramural spread ≤5 mm or >5 mm resulted in markedly different survival6,7 and local recurrence rates.6
There is a wide range of CRM involvement in different series and registries,11,36 mainly related with the quality of surgery, especially in low rectal cancer and abdominoperineal resection procedures.21 However, the rates of CRM involvement are also related to the interest and training of pathologists, and series with high-quality pathological procedure are more likely to reflect the true incidence of CRM involvement.16 Assessment of CRM status should receive close attention from the surgeon at the time of surgery and subsequently from the pathologist by careful specimen dissection and histologic assessment. Thus, a feasible, reliable pathologic method in assessing CRM and DMI is necessary.
The method of CRM and DMI assessment has evolved over time. In earlier WM studies, the fresh specimen was opened along the antimesenteric border, avoiding the tumor section where possible. After fixation in 4% formalin for 48 h, the whole tumor was sliced serially and transversely at 5−10 mm intervals with a large scalpel. Thereafter, the entire tumor and surrounding mesorectum were embedded and examined using WM mega slides.1,13–15,28 However, when TME was adopted, longitudinal slices were replaced by transversal slices to provide multiple coronal sections through the tumor and the mesorectum, allowing for simultaneous assessment of the CRM and the integrity of the mesorectum.2,4,26
Although most studies dealing with CRM assessment refer to Quirke’s protocol (Quirke 1986), few specify the use of WM slices. The availability of microtomes with mega-cassette clamps is scarce, and training is required for proper technical use. Moreover, the size of the slice is another limitation when it is over the mega-cassette area. This situation is not rare in cases of surgery beyond TME and pelvic exenterations, which makes the use of SB necessary37,38 as the correct orientation of the specimen and cassettes are crucial to get a reliable information for Multidisciplinary Committee decisions. The mesorectal fat amount in WM sections is another disadvantage because the fatty thickness presents difficulties when the mega-blocks are cut in microtomes. The main advantages of WM sections are their easier inclusion in mega-cassettes and the anatomic view of the specimen, which makes spatial orientation easier.
The main limitation of this study is that comparative WM and SB histopathological assessments were not performed in the same slice because 2 different contiguous slices would have been necessary to compare both dissection techniques. Despite this limitation, we observed minimal differences regarding measurement values. Moreover, only one patient had a positive CRM, and therefore the study is not powered to compare the 2 techniques to determine CRM status. Finally, due to the relatively small sample size, it was not possible to perform sub analyses for specific groups of patients (for example, patients who had undergone preoperative chemo-radiotherapy).
In conclusion, both gross dissection techniques (whole-mount vs small blocks) are equivalent and reliable to assess the distance to CRM and the depth of mesorectal invasion into the mesorectal fat in rectal cancer staging.
Compliance of ethical standardsThe authors declare the compliance of ethical standards in research.
Conflicts of interestThe authors declare that they have no conflicts of interest.