Endoscopic resection offers advantages over surgical resection for early colorectal cancer (ECC). However, there might be a presumed risk of recurrence. We aimed to determine the risk of recurrence after endoscopic removal of ECC.
MethodsA single-centre series of endoscopic resections for ECC. Patients were stratified according to four risk factors: positive resection margins, Haggitt 4, lymphatic/vascular invasion and tumour budding.
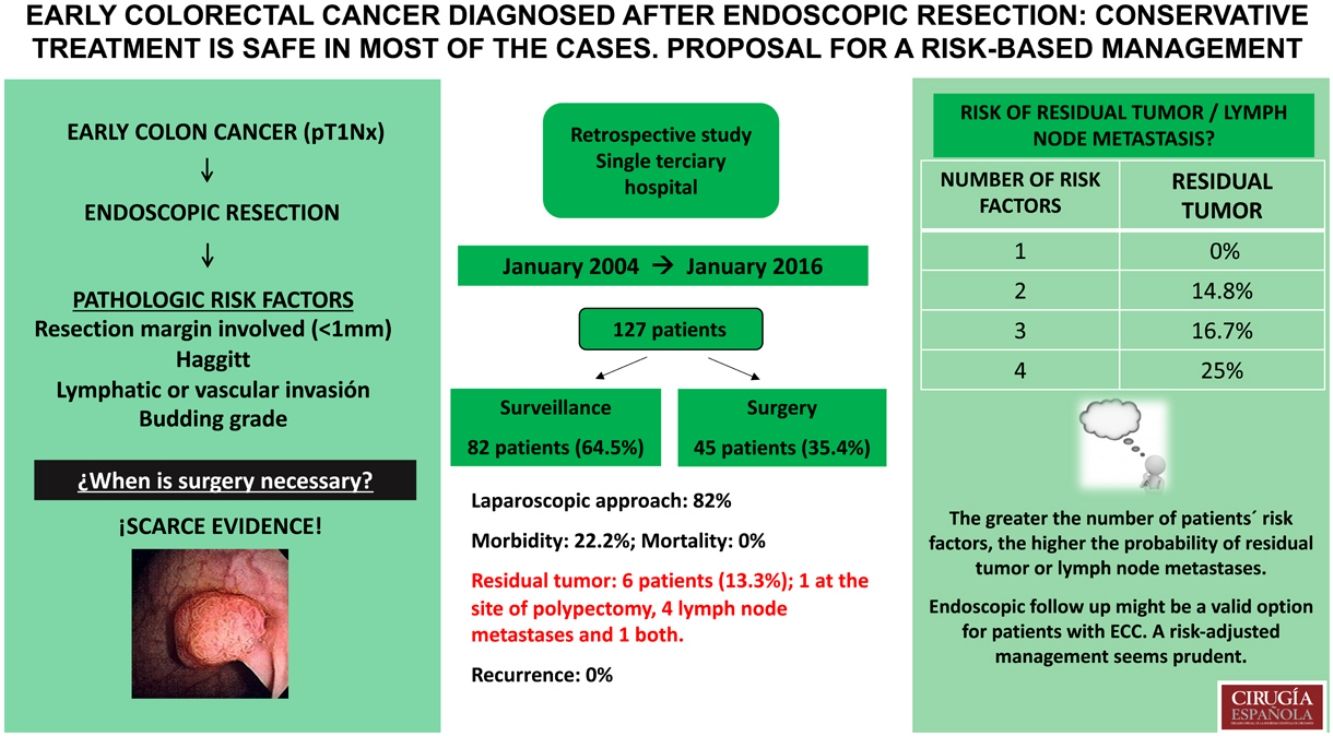
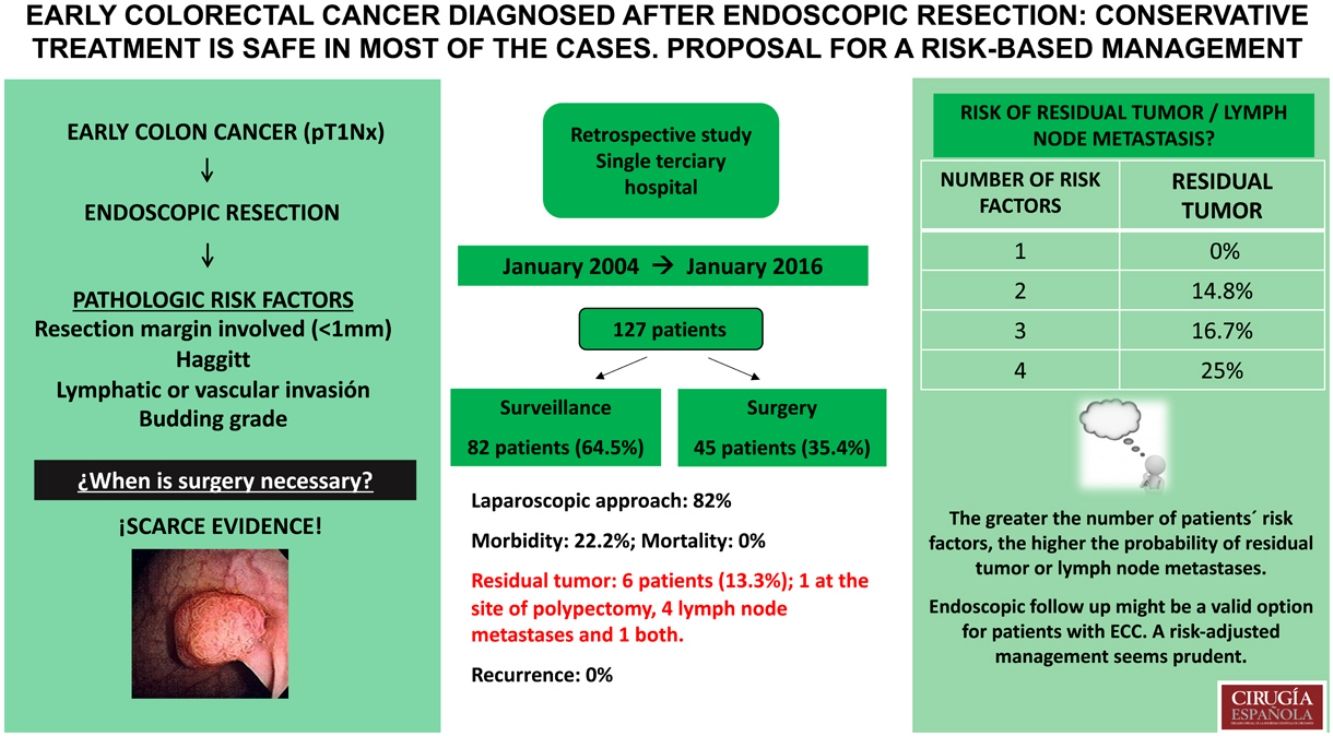
ResultsWe included 127 patients. Haggitt classification was grade 4 in 54.0%. Positive margins were found in 43 (33.9%), 16 (12.6%) had lymphatic or vascular invasion, and 5 (4.0%) had high grade budding. In 82 (64.5%) endoscopic excision was the definitive treatment, 45 (35.4%) underwent surgery. Six patients (13.3%) had residual tumour on specimen and/or node metastases. Postoperative complications occurred in ten (22.2%). At a median follow-up of 63 months, none of the 82 patients treated with endoscopic resection alone had recurrence. After stratifying patients according to risk factors, those who had residual tumour also had ≥2 risk factors.
ConclusionsEndoscopic follow up might be a valid option for patients with ECC. A risk-adjusted management seems prudent.
La resección endoscópica ofrece claras ventajas frente a la cirugía en el tratamiento del cáncer de colon inicial (ECC). Sin embargo, existe un riesgo de recurrencia tanto a nivel del lecho de polipectomía como a nivel ganglionar. El objetivo del estudio es determinar el riesgo de recurrencia tras la resección endoscópica del ECC.
MétodosSerie retrospectiva unicéntrica de resecciones endoscópicas de ECC. Se analizaron cuatro factores de riesgo en la pieza de polipectomía: el margen de resección afecto, Haggitt 4, invasión linfovascular y la presencia de budding tumoral.
ResultadosSe incluyeron 127 pacientes: Haggitt 4 en el 54%, margen de resección afecto en el 33,9%, infiltración linfática o vascular en el 12,6% y budding tumoral de alto grado en el 4%. En 82 pacientes (64,5%), la resección endoscópica fue el tratamiento definitivo. En 45 (35,4%) se realizó una colectomía oncológica. Seis pacientes (13,3%) presentaron tumor residual en el lecho de la polipectomía y/o a nivel de los ganglios linfáticos. La morbilidad postoperatoria fue del 22% y la mortalidad del 0%. Tras un seguimiento medio de 63 meses, ninguno de los 82 pacientes del grupo de polipectomía presentó recurrencia tumoral. Tras dividir a los pacientes según el número de factores de riesgo presentes, aquellos que presentaron tumor residual en la pieza de colectomía presentaban a su vez ≥ 2 factores de riesgo.
ConclusionesEl seguimiento endoscópico puede ser una opción válida en los pacientes con ECC. El manejo de estos pacientes debe ajustarse al riesgo individual, en función del número de factores de riesgo.
Early colorectal cancer (ECC) is defined as a carcinoma with invasion limited to the submucosa. It corresponds to the T1NXM0 stage according to the 8th TNM edition of the American Joint Committee on Cancer (AJCC) staging system.1
The benefits of endoscopic resection over surgical resection include a less invasive approach and fewer complications. However, it has been associated with a higher risk of recurrence due to incomplete tumour resection and lymph node metastases. The risk of lymph node metastases ranges from 6 to 17% between different series, therefore the management of these patients remains controversial, with two alternative therapeutic options: endoscopic resection and oncological surgical resection.2,3
Multiple risk factors have been associated with a higher recurrence rate and lymph node metastases, most important being poor differentiation, presence of tumour budding, the depth of tumour invasion into the submucosal layer or lympho-vascular invasion. Currently the evaluation of these factors is helpful when deciding the correct line of treatment for these patients.3,4
The primary aim of this study was to determine the risk of recurrence in a consecutive series of patients who underwent endoscopic resection for ECC. Secondary aim consisted of the rate of residual tumour and lymph node invasion in those who underwent surgical excision after primary endoscopic resection.
Materials and methodsStudy design, inclusion and exclusion criteriaA longitudinal retrospective study was performed in a single tertiary centre to identify patients with ECC (T1NXM0) diagnosed after endoscopic resection of a colonic polyp with curative intention between January 2004 and January 2016, regardless of whether the definitive treatment was surgery or not. Patients with macroscopically incomplete endoscopic resection of the polyp or piecemeal resection, synchronous colorectal cancer at the time of the colonoscopy or in the previous 5 years, or cancer of another origin in the previous 5 years, were excluded from the analysis. Patients with no follow-up available, were also excluded.
The decision to perform an endoscopic resection as initial treatment in colorectal polyps is based on agreed morphological and visual criteria.5–7 When profound submucosal invasion was suspected, endoscopic resection was not performed, and biopsy plus endoscopic tattoo were carried out for subsequent surgical resection. The selected endoscopic excision method in all of the patients was endoscopic mucosal resection.
After endoscopic resection, those patients with pT1 cancers and histological risk factors were discussed at a colorectal multidisciplinary committee. The following parameters were considered as risk factors for local recurrence or lymph node involvement:
- 1.
resection margin involved (<1mm),
- 2.
Haggitt 4,
- 3.
lymphatic or vascular invasion,
- 4.
High grade budding.
In case of presenting at least one of the risk factors, the patients were advised to undergo surgical excision. Then, based both on the patients’ health status and preference, the decision was made to perform either endoscopic/radiological follow-up or oncologic surgical resection.
The histological examination of both polypectomies and surgical specimens was performed by a dedicated pathologist of the colorectal cancer multidisciplinary team.
According to our protocol, surveillance after endoscopic resection in ECC was performed with periodic colonoscopies to rule out local recurrence, as well as with imaging tests (ultrasound or abdominal computed tomography, CT) to rule out distant metastases. The first colonoscopy was performed 6 months after endoscopic resection, the next one at 12 months, and annually thereafter. The first CT was performed at 6 months and then annually. Patients who underwent surgical resection were followed up in the outpatient clinic with regular serum CEA (every 6–12 months), colonoscopy at 1, 3 and 5 years after surgery, and CT chest, abdomen and pelvis at 6 and 12 months and then annually until completing 5 years of follow-up.
Data collectionData of interest included patient characteristics, tumour characteristics (size, morphology, location), endoscopic treatment characteristics (method, complications), histological variables of polypectomies (Haggitt, tumour budding, lympho-vascular infiltration, resection margin), surgical treatment characteristics (procedure, laparoscopic or open approach, length of hospital admission, readmission rate, morbidity and mortality), histological variables of the surgical specimen, and follow-up variables.
Tumour budding was defined as an isolated cancer cell or a cluster composed of fewer than 5 cells in the invasive frontal region.8 The number of buddings is counted in a single field. Depending on the number of buddings, the grade of budding is defined as low (0–4) or high (>5).8 Lympho-vascular invasion was defined as the presence of tumour cells within the endothelial-lined spaces.9
Statistical analysisQualitative variables are described with the absolute frequency and the percentages, n (%). Quantitative variables are expressed with the median (range). Comparison of categorical and continuous variables were performed by means of Fisher's exact test and Mann–Whitney test, respectively. P value <0.05 were considered statistically significant.
The study adhered to the Declaration of Helsinki (1964) and patients signed written informed consent before receiving the procedures.
ResultsOverall, 127 patients diagnosed with ECC after endoscopic polyp resection were included in our analysis. The median age of patients was 63 (32–87) years, 59% of the patients were men.
The polyps varied in their morphology: 63.1% were pedunculated, 25.4% sessile and the remainder pseudo-pedunculated. The most common locations were the sigmoid and descending colon (75.5%), followed by the rectum (17.3%) and the ascending and transverse colon (6.3 and 0.8% respectively) (Table 1).
Characteristics of the patients.
| Total. n=127 (%) | Not operated on n=82 (64.6%) | Operated on n=45 (35.4%) | P value | |
|---|---|---|---|---|
| AGE (years), median (range) | 63 (32–87) | 65 (47–87) | 61 (32–79) | 0.004 |
| Polyp size (mm), median (range) | 15 (5–50) | 15 (5–50) | 15 (6–40) | 0.828 |
| Polyp location, n (%) | 0.388 | |||
| Ascending colon | 8 (6.3%) | 4 (6.0%) | 4 (8.8%) | |
| Transverse colon | 1 (0.8%) | 1 (1.2%) | 0 (0.0%) | |
| Descending & sigmoid colon | 96 (75.6%) | 60 (72.3%) | 36 (81.8%) | |
| Rectum | 22 (17.3%) | 17 (20.5%) | 5 (11.4%) | |
| Polyp morphology, n (%) | 0.109 | |||
| Sessile | 31 (25.4%) | 16 (20.8%) | 15 (33.3%) | |
| Pedunculated | 77 (63.1%) | 54 (70.1%) | 23 (51.1%) | |
| Pseudo-pedunculated | 14 (11.5%) | 7 (9.1%) | 7 (15.6%) | |
| Haggitt, n (%) | <0.001 | |||
| Haggitt 1 | 28 (22.2%) | 26 (31.7%) | 2 (4.5%) | |
| Haggitt 2 | 13 (10.3%) | 12 (14.6%) | 1 (2.3%) | |
| Haggitt 3 | 17 (13.5%) | 14 (17.1%) | 3 (6.8%) | |
| Haggitt 4 | 68 (54.0%) | 30 (36.6%) | 38 (86.4%) | |
| Resection margins, n (%) | <0.001 | |||
| Positive | 43 (33.9%) | 10 (12.2%) | 33 (73.3%) | |
| Negative | 76 (59.8%) | 68 (82.9%) | 8 (17.8%) | |
| Doubtful | 8 (6.3%) | 4 (4.9%) | 4 (8.9%) | |
| Vascular invasion, n (%) | 0.007 | |||
| Absent | 114 (89.8%) | 78 (95.1%) | 36 (80.0%) | |
| Present | 13 (10.2%) | 4 (4.9%) | 9 (20.0%) | |
| Lymphatic invasion, n (%) | 0.003 | |||
| Absent | 111 (87.4%) | 77 (93.9%) | 34 (75.6%) | |
| Present | 16 (12.6%) | 5 (6.1%) | 11 (24.4%) | |
| Differentiation grade, n (%) | 0.064 | |||
| G1 | 55 (43.3%) | 38 (46.3%) | 17 (37.8%) | |
| G2 | 66 (53.6%) | 44 (53.6%) | 24 (53.3%) | |
| G3 | 3 (2.4%) | 0 (0.0%) | 3 (6.7%) | |
| Budding, n (%) | 0.001 | |||
| Absent | 96 (76.2%) | 70 (85.4%) | 26 (59.1%) | |
| Low grade | 25 (19.8%) | 12 (14.6%) | 13 (29.5%) | |
| High grade | 5 (4.0%) | 0 (0.0%) | 5 (11.4%) | |
| Local recurrence, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Distant metastases, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Follow up (years), median (range) | 5.25 (2.7–7.2) | 5.41 (3.9–7.2) | 5.04 (2.7–6.6) | 0.005 |
Pathology of the resected specimen revealed that 54.0% of them corresponded to grade 4 of the Haggitt classification, 13.5% to grade 3, 10.3% to grade 2 and 22.2% to grade 1.
In 43 cases (33.9%) the resected specimen presented positive resection margins. The margin was doubtful in 8 cases (6.3%). Only 16 (12.6%) patients presented lymphatic or vascular invasion and 30 (23.8%) had tumour budding, 25 low grade and 5 high grade. 53.6% of the cases were moderately-differentiated tumours, 43.3% well-differentiated and 2.4% poorly-differentiated (Table 1).
Out of 127 patients included, in 82 (64.5%) endoscopic excision was the definitive treatment, whilst 45 (35.4%) were offered surgery. Three patients required emergency surgery due to colonic perforation during colonoscopy. Sigmoidectomy was the most commonly performed procedure (57.7%). A laparoscopic approach was used in 37 (82%) cases, with a conversion rate of 4.4% (Table 2).
Characteristics of the patients who underwent surgery after cancer diagnosis following endoscopic polypectomy (n=45).
| Variable | |
|---|---|
| Technique, n (%) | |
| Sigmoidectomy | 26 (57.7%) |
| Anterior rectal resection | 10 (22.2%) |
| Left hemicolectomy | 5 (11.1%) |
| Right colectomy | 3 (6.8%) |
| Subtotal colectomy | 1 (2.2%) |
| Approach, n (%) | |
| Laparoscopic | 37 (82.2%) |
| Conversion rate | 2 (4.4%) |
| Residual tumour, n (%) | 2 (4.4%) |
| Lymph nodes resected median (range) | 8 (0–24) |
| N0 | 40 (88.9%) |
| N1a | 2 (4.4%) |
| N1b | 3 (6.7%) |
| Admission length,(days) median (range) | 6 (4–29) |
| Readmission, n (%) | 4 (8.9%) |
| Morbidity, n (%) | 10 (22.2%) |
| Mortality, n (%) | 0 (0) |
The postoperative analysis of the resected specimens showed that 6 patients (13.3%) had residual tumour at the site of polypectomy (2 cases, 4.4%) and/or lymph node metastases (5 cases, 11% – 2 of N1a stage, 3 of N1b stage according to the TNM). The median number of the resected lymph nodes was 8 (0–24). Postoperative complications occurred in 10 patients (22.2%), including 6 (13.6%) anastomotic leaks. The median length of hospital stay was 6 (4–29) days. The mortality rate was nil (Table 2).
None of the 82 patients who received endoscopic resection had either local recurrence or metastases. The median follow-up in this subgroup was 65 (47–87) months.
Only one of the operated patients did not have any of the risk factors and was operated due to colonic perforation during colonoscopy. 21.9% of the patients with a single risk factor surgical treatment was operated and none of them showed residual tumour or lymph node metastases in the anatomopathological analysis. 82.2% of the patients who underwent surgery had 2 or more risk factors. The greater the number of patients’ risk factors, the higher the probability of residual tumour or lymph node metastases. Correspondingly, it is 0% in patients with 0 and 1, 14.8% in patients with 2, 16.7% in patients with 3 and 25.0% in patients with 4 risk factors (Table 3).
Management of patients according to the number of risk factors in the polypectomy specimen (positive resection margins, Haggitt 4, lymphatic/vascular invasion, tumour budding). The patients who underwent surgery are divided into 2 groups according to whether or not residual tumour was found in the surgical specimen.
| Outcome | Number of risk factors | Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Not operated on | 44 (97.9%) | 25 (78.1%) | 11 (28.9%) | 2 (25.0%) | 0 (0) | 82 |
| Operated on | 1 (2.1%) | 7 (21.9%) | 27 (71.1%) | 6 (75.0%) | 4 (100.0%) | 45 |
| No residual tumour | 1 (100.0%) | 7 (100.0%) | 23 (85.2%) | 5 (83.3%) | 3 (75.0%) | 39 |
| Residual tumour | 0 (0) | 0 (0) | 4 (14.8%) | 1 (16.7%) | 1 (25.0%) | 6 |
| Total | 45 | 32 | 38 | 8 | 4 | 127 |
The aim of endoscopic polypectomy is to achieve complete excision of the lesion with negative margins (both lateral and vertical). It is currently validated as a curative treatment in ECC (T1NXM0), as long as the tumour infiltration does not exceed 1mm (m/sm1) of the submucosa, presents neither lymphatic nor vascular invasion, is well o moderately differentiated (G1, G2) and has a low tumour budding component.9
The obvious benefits of the less invasive endoscopic treatment come with the cost of the presumed higher incidence of incomplete resection and lymph node infiltration, the incidence of the last one varying between 6 and 17% according to different series.2,3
Multiple risk factors possibly influencing the oncological outcomes of an endoscopic polypectomy (tumour recurrence) have been analysed, i.e. positive resection margins, depth of submucosa infiltration, lymphatic and vascular invasion, poor differentiation and tumour budding presence.10–13
The risk of residual tumour in patients submitted to endoscopic polypectomy with negative resection margins (>1mm) is lower than 2%.11 On the other hand, a positive resection margins increases this risk up to 21–33%.12,13 In our series, the percentage of positive resection margins was 33.9% and doubtful resection margins were found in 6.3%. Ten patients with positive resection margins were managed with endoscopic surveillance with biopsies, and none of them had signs of local recurrence or residual tumour. Among the 33 patients operated on due to positive resection margins, only one had evidence of residual tumour in the surgical specimen.
Patients with pedunculated polyps grade 1, 2 and 3 according to Haggitt classification show a risk of lymph node metastases lower than 1%. This risk increases up to 6.2% in Haggitt 4 lesions.14 The lymphatic involvement in sessile polyps can be assessed with the Paris Classification, accounting for an estimated 1% risk in sm1 polyps, while in sm3 lesions it can be as high as 15%.5 Although the grade of submucosa infiltration is an established risk factor for tumour recurrence, insomuch that some authors consider infiltration deeper than 1mm as an indication for surgery, other authors regard this factor to be of less importance compared with other variables, with low recurrence rate even when no surgical rescue is performed.8,15 In our series, 36.6% of the patients with Haggitt 4 lesion were not operated on, and none of them showed tumour recurrence in the follow up.
Vascular and lymphatic invasion was present in 12.6% of our cases. This factor is associated with higher risk of lymph node metastases, and most authors recommend surgical excision.15–18
Tumour budding has been correlated with a higher risk of lymph node metastases, up to 42.1%. As this rarely occurs as an isolated risk factor, it is difficult to establish its actual impact on patient prognosis, most authors considering it an indication for surgery.17–19 In our series, 5 patients presented high grade budding, all of them had at least 3 or more risk factors. The 5 patients were operated and one showed lymph node metastases in the surgical specimen.
According to the latest published guidelines,20 the presence of one or more of the mentioned risk factors in the polypectomy specimen represents an indication for surgical excision. The grade of the evidence presented being low, at present, a careful evaluation of the underlined determinants will help guiding towards a correct line of treatment – whether it is an expectant attitude with endoscopic controls or a surgical resection.
In our series, none of the patients who underwent surgery with only one risk factor had residual tumour or lymph node involvement at pathology analysis. On the other hand, such findings were found in 14.8% of patients with two, 16.7% of patients with three, and 25.0% of patients with four risk factors. The vast majority of patients with only one risk factor had follow-up with endoscopy, without showing signs of neither local nor distant recurrence by the end of the follow up.
Our study has several limitations. It is a retrospective study and we were not able to evaluate the depth of submucosa infiltration. However, it was performed in a tertiary centre with a dedicated multidisciplinary team and expertise in the management of the condition, making results reproducible. Our information could be useful along with other biomarkers of CRC aggressiveness and progression.
ConclusionThe initial management of ECC diagnosed after a prior endoscopic polypectomy can vary from endoscopic controls to oncologic surgical resection with adequate lymphadenectomy. The choice relies not only on the results of the polypectomy specimen exam, but also on patient health status and tumour location. The risk of recurrence has to be weighed against the risks of surgery and general anaesthesia.
The low recurrence rate after an initial endoscopic polypectomy suggests that endoscopic follow-up might be a valid option for patients with none or one risk factor. Surgery seems to be the ideal option to treat patients with two or more risk factors due to substantial risk of residual tumour or lymph node metastases.
FundingAll authors have no source of funding.
Conflict of interestAll authors have not conflict of interest.