The great majority of breast cancer (BC) cases are diagnosed in women who have no known family history of the disease and are not carriers of any risk mutation. During the past few decades an increase in the number of contralateral prophylactic mastectomy (CPM) has been produced in these patients. The CBCRisk model calculates the absolute risk of suffering from contralateral breast cancer (CBC); thus, it can be used to counselling patients with sporadic breast cancer.
MethodAn observational, retrospective study including sporadic breast cancer patients treated with contralateral prophylactic mastectomy has been conducted between 2017 and 2019. A descriptive and comparative study with one variation of logistic regression has been carried out in order to identify predictive factors of occult tumors (OT) and medium/high risk damage (MHRD). Evaluation of the CBCRisk model published in 2017 and different limit values for the CPM recommendation.
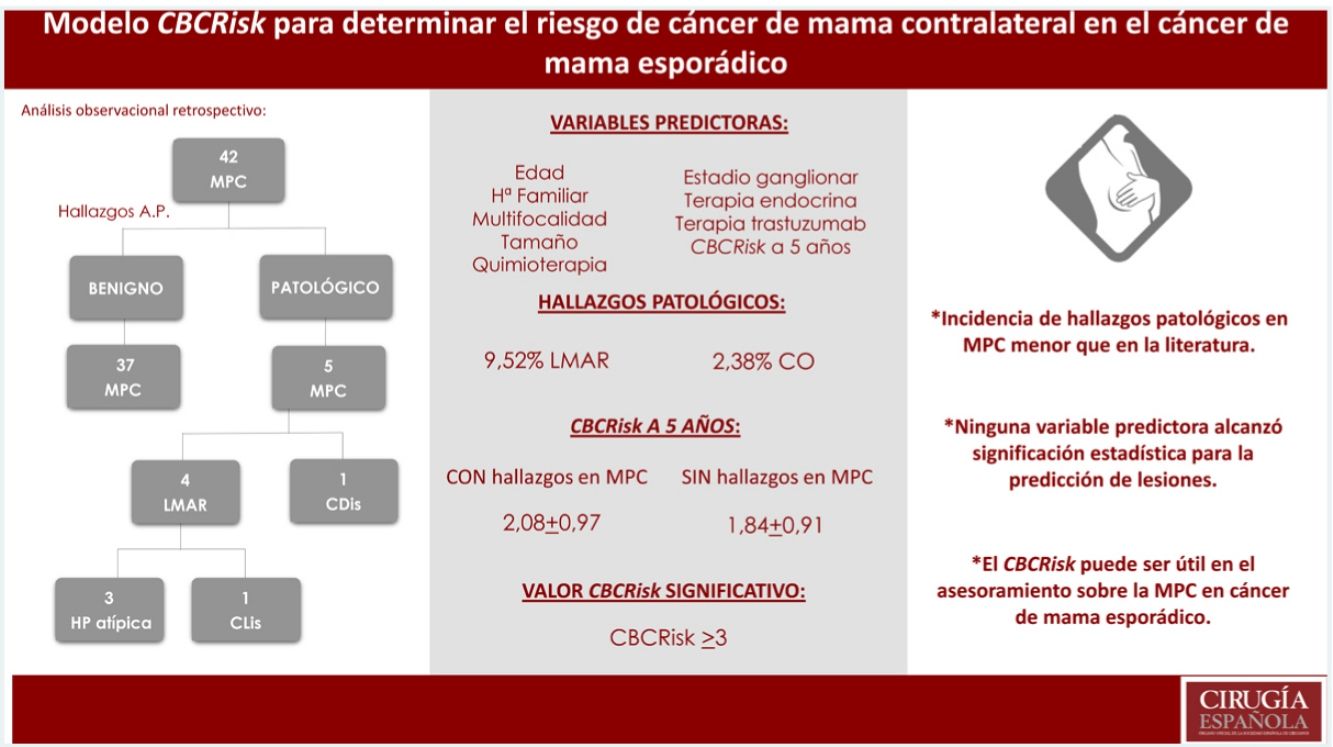
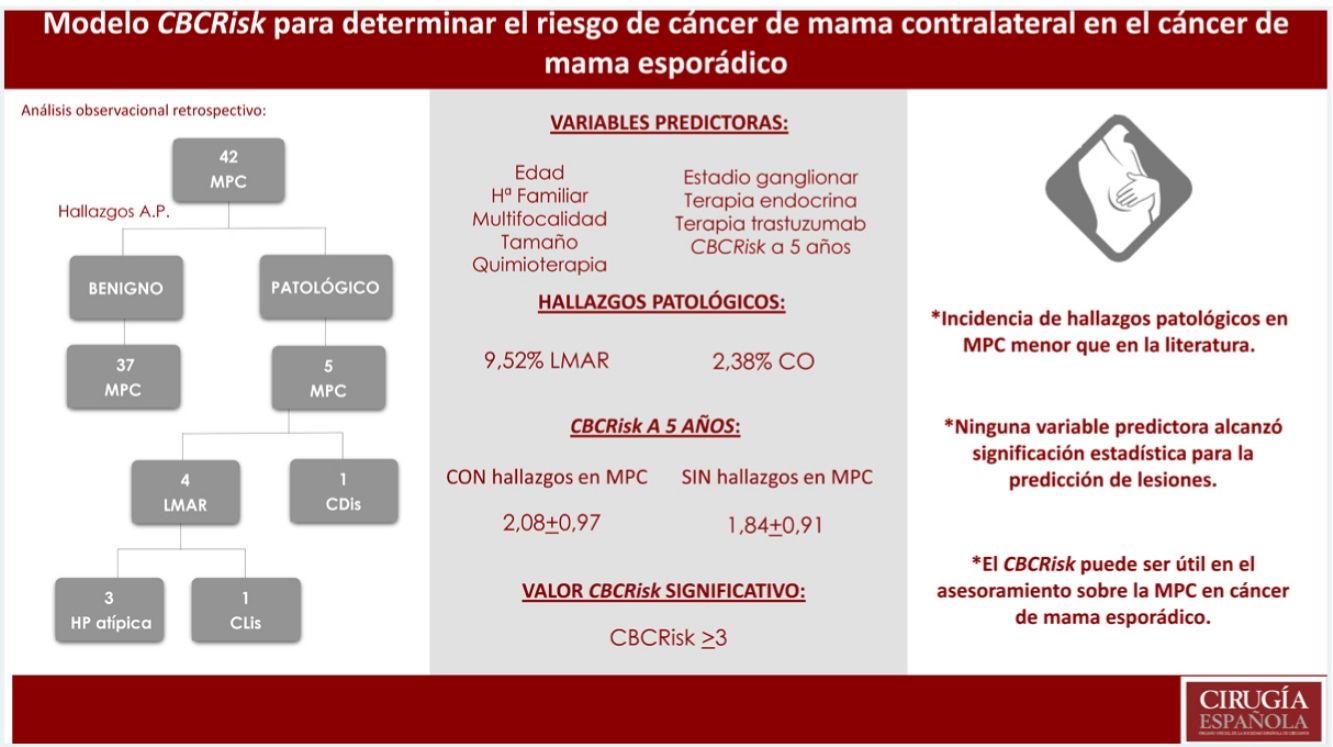
Results42 patients were selected. Incidence of MHRD and OT was lower than that described in the literatura (9.52%MHRD, 2.38%OT). None of the evaluated variables reached statistical significance for predicting injuries. The average value of CBCRisk 5 years ahead found in patients with pathological findings was 2.08 (DE 0.97), higher than the average value of the whole group (1.87 ± 0.91) and the subgroup without pathological findings (1.84 ± 0.91). Only values >3 for CBCRisk were considered statistically significant (P = .04) for the prediction of histological lesions.
ConclusionPatients with sporadic breast cancer should be adequately informed about the estimated risks and benefits of undergoing a contralateral prophylactic mastectomy. The CBCRisk may be useful for the counseling of these patients, but it requires validation in larger and prospective cohorts.
La mayoría de los cánceres de mama (CM) se diagnostican en mujeres sin antecedentes familiares y no portadoras de mutaciones de riesgo. En las últimas décadas se ha producido un aumento de mastectomías profilácticas contralaterales (MPC) en estas pacientes. El CBCRisk es un modelo que calcula el riesgo absoluto de cáncer de mama contralateral (CMC) y pretende servir para el asesoramiento de pacientes con CM esporádico sobre la MPC.
MétodoAnálisis observacional retrospectivo de pacientes con un cáncer de mama esporádico sometidas a MPC durante 2017−2019. Análisis descriptivo, comparativo y de regresión logística univariante para identificar factores predictivos de LMAR y/o CMC oculto. Evaluación del modelo CBCRisk publicado en 2017 y distintos valores límite para la recomendación de MPC.
ResultadosSe seleccionaron 42 pacientes. Incidencia de LMAR y CO menor que la descrita en la literatura (9.52%LMAR, 2,38%CO). Ninguna de las variables evaluadas alcanzó significación estadística para la predicción de lesiones. El valor de CBCRisk a 5 años medio en pacientes con hallazgos patológicos fue de 2.08(DE 0.97), superior al CBCRisk medio del conjunto (1.87 ± 0.91) y del subgrupo de MPC sin hallazgos patológicos (1.84 ± 0.91). Sólo el CBCRisk>3 resultó significativo (P = .04) para la predicción de hallazgos patológicos.
ConclusiónLas pacientes con CM esporádico deben ser adecuadamente informadas de los riesgos y beneficios estimados de la MPC. El CBCRisk puede ser útil para el asesoramiento de estas pacientes, pero precisa validación en cohortes más amplias y prospectivas.
Contralateral prophylactic mastectomy (CPM) has been evaluated as a strategy for reducing the risk of contralateral breast cancer (CBC) in patients with sporadic breast cancer (BC) (ie, no mutations in the main genes associated with hereditary breast cancer, and no strong family history)1.
CPM is the therapeutic strategy that provides the greatest reduction in the risk of CBC. It reduces the need for follow-up controls along with the concern and anxiety of patients, while providing benefits in terms of symmetrization. However, it is a compromising procedure: it is aggressive and irreversible; it doubles the risk of surgical complications2; it can delay the administration of adjuvant therapies; it often requires other procedures (mainly due to the association of some type of breast reconstruction); it can be associated with chronic pain3 and may negatively influence the mental and sexual health of patients4. Furthermore, the benefits of CPM in terms of survival of women who are not carriers of BRCA mutations has not been clearly demonstrated, except perhaps for women under the age of 49 with triple negative tumors5.
Consensus indications1 for CPM include a history of supradiaphragmatic radiation before the age of 30 and a demonstrated BRCA 1/2 mutation. It can be considered in the case of CHEK2/PTEN/p53/PALB2/CDH1 mutation carriers, patients with a strong family history without demonstrated risk mutations, or in the case of significant asymmetry after unilateral mastectomy (with or without reconstruction).
In recent decades, we have witnessed an increase in the performance of CPM in patients with sporadic BC, more notable among the US population than in the European population due to social and cultural factors that have not yet been clarified6,7. This fact is paradoxical as the early diagnosis of BC together with improved adjuvant therapies has meant that, on the one hand, the possibilities of breast-conserving techniques has increased (expanded indications for breast-conserving surgery), while the recurrence, mortality and incidence of CBC have decreased8.
Some authors attribute this increase in CPM to patients overestimating the risk of CBC, as well as the generalized access to immediate reconstruction1,6.
Until the publication of the absolute risk predictive model (CBCRisk9) in 2017, there was no useful quantitative tool for individual CBC risk assessment in women with sporadic unilateral BC. This model calculates the absolute risk of CBC by periods, through the combination of eight risk factors: age at diagnosis of the first BC, antiestrogenic therapy, first-degree family history of BC, previous moderate/high-risk lesions (MHRL), estrogen receptor status, breast density at diagnosis, type of primary tumor, and age at birth of the first child. The tool is specially designed for women with sporadic unilateral BC, as it does not include information on risk of mutations.
Subsequently, the ability of the model to discriminate between women with high/low risk of CBC was evaluated in two independent cohorts10, but the follow-up data only allowed this to be done for a period of three to five years. The authors concluded that, although there are differences depending on the prevalence of BC, the characteristics of the cohort and the parameters coded as “unknown”, the model can be useful for individual counseling in routine clinical practice.
The objective of this study is to evaluate whether the CBCRisk is able to identify patients with a greater probability of presenting MHRL or occult malignancy in the contralateral breast, thereby making it possible to identify patients who would obtain the greatest benefit from CPM.
MethodsStudy populationThe study has included women with sporadic unilateral BC who underwent CPM between January 2017 and March 2019, conducted by a single Breast Unit.
The following exclusion criteria were applied:
- -
Age <18 years and >88 years (to adjust the sample to the CBCRisk model, which is specially designed for women aged 18–88)
- -
Patients with breast cancer and proven high-risk genetic mutations
- -
Patients without breast cancer treated with bilateral risk-reducing mastectomy
- -
Patients with bilateral breast cancer
- -
Patients with lobular carcinoma in situ (LCIS)
- -
Patients with non-ductal or non-lobular tumors
- -
Patients treated with supradiaphragmatic radiation therapy before the first diagnosis of BC
Two groups of variables were retrospectively analyzed:
- 1
Demographic variables necessary for the calculation of the CBCRisk:
- -
Age at diagnosis of the initial BC
- -
Antiestrogenic therapy for the treatment of the primary tumor
- -
History of BC in first-degree relatives
- -
Moderate/high-risk lesions (MHRL) prior to the diagnosis of BC
- -
Breast density at diagnosis (according to the BI-RADS score: predominantly fatty, scattered areas of density, heterogeneously dense, and extremely dense patterns)
- -
Age at birth of the first child (<30 years or nulliparity, 30–39, ≥40)
- -
Primary tumor type (pure non-invasive cancer [DCIS], pure invasive, mixed-DCIS, and invasive)
- -
- 2
Clinicopathological variables:
Tumor-related
- -
Tumor size (≤2 cm, 2−5 cm, ≥5 cm)
- -
Multifocality
- -
Lymph node stage
- -
Adjuvant chemotherapy
- -
Estrogen receptor positivity (hormone therapy)
- -
HER2 positivity (trastuzumab therapy)
- -
Findings of CPM in samples
- -
Ductal or lobular atypical hyperplasia (AH) and lobular carcinoma in situ (LCIS) were considered moderate/high-risk lesions (MHRL).
Statistical analysisPathological findings in the CPM specimens were compared over 5 years with the CBCRisk model, using the online calculator (available at https://cbc-predictor-utd.shinyapps.io/CBCRisk/). The CBCRisk was considered exclusively after five years.
First of all, we conducted a descriptive analysis of the variables under study: relative and absolute frequencies (qualitative) and mean and standard deviation (SD) (quantitative). We evaluated the relationship between qualitative variables using the chi-squared test. To compare means between two independent groups, the Mann-Whitney U test or Student’s t test was used, according to normality criteria. We also conducted a univariate logistic regression analysis to identify predictors of MHRL and/or occult contralateral breast cancer. A P value <.05 was considered statistically significant, and the SPSS 22.0 statistical program was used throughout. This study was approved by our hospital Ethics Committee (Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain).
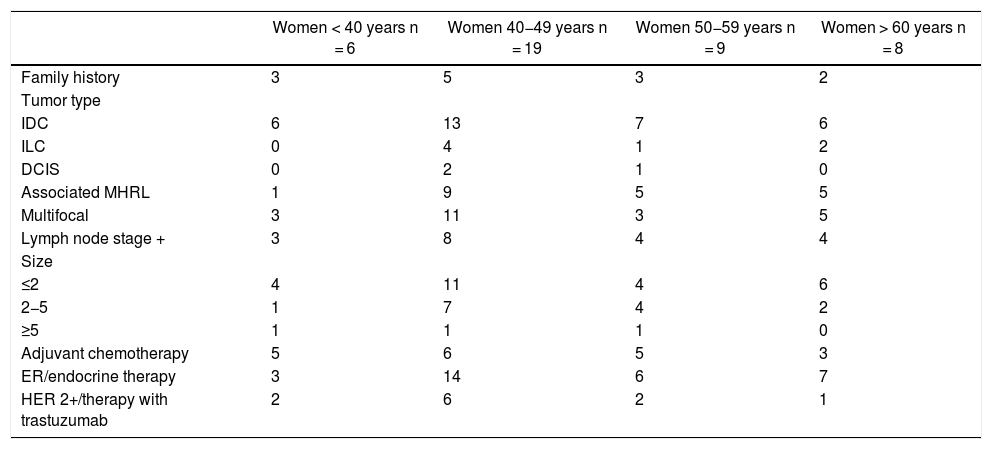
ResultsDescription of the study populationA total of 42 women underwent bilateral mastectomy for sporadic unilateral breast cancer. Table 1 shows their demographic and clinicopathological characteristics.
Demographic and clinical-pathological data.
| Women < 40 years n = 6 | Women 40−49 years n = 19 | Women 50−59 years n = 9 | Women > 60 years n = 8 | |
|---|---|---|---|---|
| Family history | 3 | 5 | 3 | 2 |
| Tumor type | ||||
| IDC | 6 | 13 | 7 | 6 |
| ILC | 0 | 4 | 1 | 2 |
| DCIS | 0 | 2 | 1 | 0 |
| Associated MHRL | 1 | 9 | 5 | 5 |
| Multifocal | 3 | 11 | 3 | 5 |
| Lymph node stage + | 3 | 8 | 4 | 4 |
| Size | ||||
| ≤2 | 4 | 11 | 4 | 6 |
| 2−5 | 1 | 7 | 4 | 2 |
| ≥5 | 1 | 1 | 1 | 0 |
| Adjuvant chemotherapy | 5 | 6 | 5 | 3 |
| ER/endocrine therapy | 3 | 14 | 6 | 7 |
| HER 2+/therapy with trastuzumab | 2 | 6 | 2 | 1 |
IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; DCIS: ductal carcinoma in situ; ER: estrogen-receptor; HER 2+: human epidermal growth factor receptor 2.
The mean age of the group was 48.61 years (SD 10.56). Only 13 (30.95%) had a family history of BC. The most frequent type of tumor in the sample in all age groups was invasive ductal carcinoma (IDC) (76.2%). The primary tumors in 22 cases were multifocal (52.38%), with associated MHRL in 20 women (47.61%). Most of the tumors were diagnosed with a size ≤2 cm (59.52%) and no lymph node extension (54.77%).
71.42% of the women underwent endocrine therapy due to the hormonal status of the primary tumor. However, only 45.23% and 26.19% received chemotherapy and trastuzumab therapy, respectively.
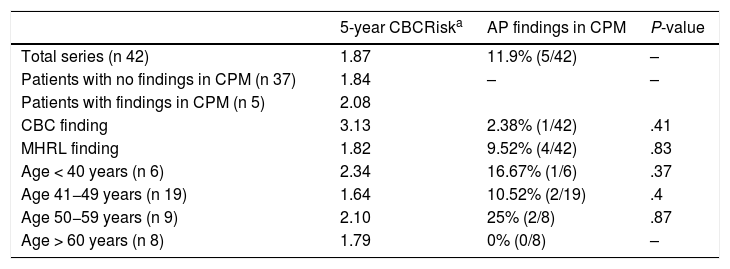
Calculated risk and pathological findings of CBC and MHRL in CPM specimensThe mean calculated CBCRisk after 5 years was 1.87 (SD 0.91), meaning that the mean absolute 5-year risk of CBC was 1.87%.
We found 4 MHRL (9.52%), 3 atypical hyperplasia (7.14%), one CLIS (2.38%) and one non-invasive cancer (DCIS) (2.38%). No invasive carcinoma was diagnosed.
The 5-year mean CBCRisk of the patients with findings of CPM in the specimens was 2.08 (SD 0.97), which was slightly higher than the mean CBCRisk of the group (1.87 ± 0.91) and of the CPM subgroup, with no pathological findings (1.84 ± 0.91). The patient who presented DCIS in the BC specimen had a calculated CBCRisk of 3.13 (this difference was not statistically significant) and her primary tumor was a T1N0 DCIS with positive hormone receptors, not requiring chemotherapy. The results are shown in Table 2.
5-year CBCRisk and anatomic-pathological findings in CPM specimens.
| 5-year CBCRiska | AP findings in CPM | P-value | |
|---|---|---|---|
| Total series (n 42) | 1.87 | 11.9% (5/42) | – |
| Patients with no findings in CPM (n 37) | 1.84 | – | – |
| Patients with findings in CPM (n 5) | 2.08 | ||
| CBC finding | 3.13 | 2.38% (1/42) | .41 |
| MHRL finding | 1.82 | 9.52% (4/42) | .83 |
| Age < 40 years (n 6) | 2.34 | 16.67% (1/6) | .37 |
| Age 41−49 years (n 19) | 1.64 | 10.52% (2/19) | .4 |
| Age 50−59 years (n 9) | 2.10 | 25% (2/8) | .87 |
| Age > 60 years (n 8) | 1.79 | 0% (0/8) | – |
AP: anatomic pathology.
It should be noted that, as in the original article, the least available variables in the patients’ medical records have been the density of the breast at diagnosis and the age at first childbirth. The model can be calculated, but cautious interpretation is required.
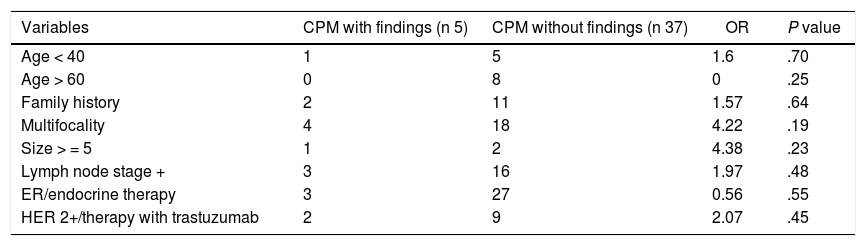
Predictive factors for CBC and MHRLA univariate analysis was performed to identify predictive factors of CBC and/or MHRL.
None of the variables analyzed reached statistical significance, probably because the sample size was small (Table 3).
Analysis of predictive factors of CBC and/or MHRL.
| Variables | CPM with findings (n 5) | CPM without findings (n 37) | OR | P value |
|---|---|---|---|---|
| Age < 40 | 1 | 5 | 1.6 | .70 |
| Age > 60 | 0 | 8 | 0 | .25 |
| Family history | 2 | 11 | 1.57 | .64 |
| Multifocality | 4 | 18 | 4.22 | .19 |
| Size > = 5 | 1 | 2 | 4.38 | .23 |
| Lymph node stage + | 3 | 16 | 1.97 | .48 |
| ER/endocrine therapy | 3 | 27 | 0.56 | .55 |
| HER 2+/therapy with trastuzumab | 2 | 9 | 2.07 | .45 |
Different cut-off points of the CBCRisk model were evaluated in the sample, finding the CBCRisk ≥3 as having the only statistically significant value (P = .04), for the prediction of pathological findings in the contralateral mastectomy specimen.
DiscussionThe risk of CBC in the general population ranges from 0.1% to 0.6% annually11. However, individual factors and factors derived from the treatment of the primary tumor have been described that could modify this risk.
In the study published by King et al.12, occult CBC was identified in 6% and MHRL in 28%. In the univariate analysis, multifocality/multicentricity was the only factor associated with CBC (OR 2.88, P = .04). However, when the authors performed the multivariate analysis, they found an association between age and progesterone receptor positivity. The authors concluded that, for the time being and until reliable predictors were identified, the low rates of occult CBC did not justify the use of CPM in moderate-risk women.
The incidence of pathological findings in our series was lower (9.52% for MHRL and 2.38% for occult BC). Invasive disease was not diagnosed, making it even more difficult to assess the true benefit of CPM for most women.
The CBCRisk model includes previously recognized risk factors reported in the literature. However, other well-known factors were rejected due to its design. The younger age at diagnosis, the presence of a first-degree family history, negative estrogen receptors, and the birth of the first child after the age of 40 were all associated with a higher incidence of CBC. Tumor size and number of affected nodes were ruled out, since it had previously been considered that a tumor size between 2 and 5 cm would increase the relative risk (RR) 1.51, and tumors >5 cm would present a RR of 1.89, and the involvement of more than 10 lymph nodes would obtain a RR 1.6213. The assessment of family history other than the first degree was ruled out, when it is known that the influence of family history responds to a complex system, however, the risk seems higher in the case of multiple antecedents, or if in addition to a first-degree relative there is also a second-degree relative13. The presence of menopause, HER-2 positivity, personal or family history of ovarian cancer, hormone replacement therapies, and body mass index were also disregarded.
In addition, it is unknown whether the combination of certain factors can exponentially increase risk. Another limitation of the model is that it does not differentiate between the type of antiestrogenic therapy administered, when it has already been shown that the risk reduction of CBC is different in the case of administering tamoxifen and aromatase inhibitors (50% vs. 70%, respectively, of risk reduction in non-carriers)14.
The score provides an ‘unknown’ option for all categories, except for the age at diagnosis and characteristics of the primary tumor. This offers the advantage of being able to calculate the CBCRisk even with little information, while assuming its limited value. However, the maximum number of variables that can be ‘unknown’ without the CBCRisk completely losing its value has not been established. This problem would be solved in the event of prospective data collection.
In addition, CBCRisk values have not been determined to recommend CPM in the general population. The ‘low risk’ values differed in the two validation cohorts (2.4 and 1.53)10, and neither of these limits was statistically significant in our sample (P = .37 and P = .64, respectively). As mentioned above, if the maximum annual risk in the general population is 0.6%, we could consider the 5-year CBCRisk ≥3 as a limit. In our sample, 11.9% of the patients had a CBCRisk ≥3, including 40% (2/5) of the pathological findings and the only diagnosed DCIS. Furthermore, in our group of patients, the CBCRisk ≥3 is the only cut-off value that reached statistical significance (P = .04).
This study has methodological limitations: a small sample size and retrospective data collection (which is why the choice of ‘unknown’ for the model calculation is not uncommon). However, we consider that the CBCRisk may be useful in routine clinical practice, since we have observed a small difference between the 5-year CBCRisk values of the patients with pathological findings in the CPM specimen, and the CBCRisk value ≥3 was statistically significant in our sample. Further studies with larger samples and a prospective design are required.
In conclusion, it is urgent to develop quantitative tools for individual assessment of the risk of CBC in women with sporadic unilateral BC to avoid unnecessary CPM. Patients must be properly informed of the risks and benefits to be expected from CPM in each individual case.
FundingThis study has received no funding.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Domingo Bretón M, Allué Cabañuz M, Castán Villanueva N, Arribas del Amo MD, Gil Romea I, Güemes Sánchez A. Modelo CBCRisk para determinar el riesgo de cáncer de mama contralateral en el cáncer de mama esporádico. Cir Esp. 2021;99:724–729.