Contralateral prophylactic mastectomy (CPM) has been reported to reduce risk of contralateral breast cancer (CBC) by at least 90%. In addition, BRCA carriers present higher risk of ipsilateral recurrence and a second primary tumor.
The aim is to evaluate risk of CBC and recurrence and to analyze predictive factors in BRCA1/2 mutation carriers and non-carriers at high-risk of hereditary breast cancer patients.
MethodsRetrospective observational study. 46 patients underwent bilateral mastectomy during 2004–2018.
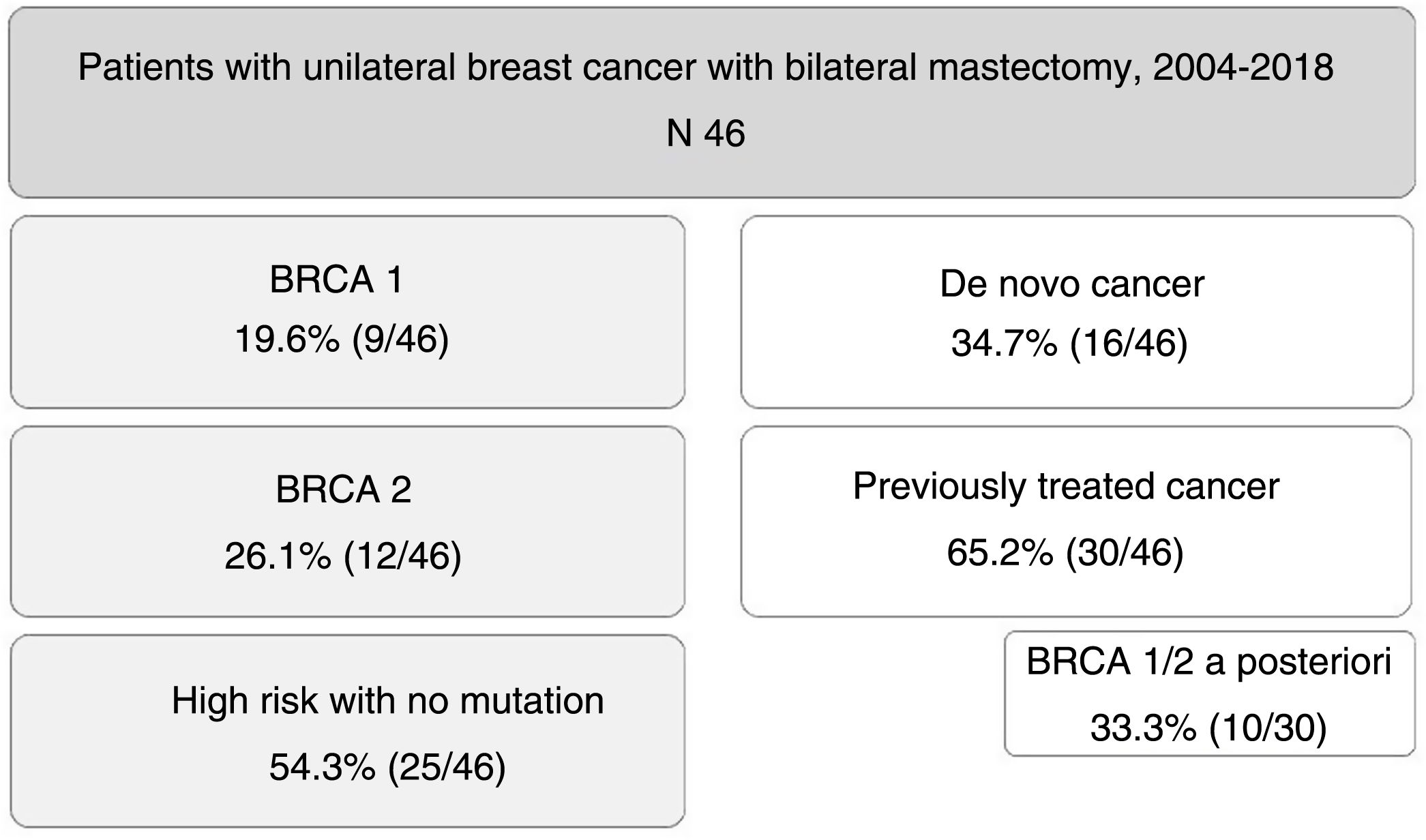
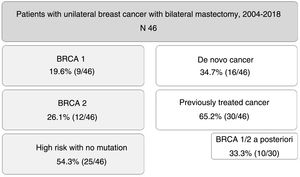
ResultsCohort comprised of 9 patients BRCA1, 12 BRCA2 and 25 at high-risk without mutation. Median follow-up was 79 months. 16 patients recently diagnosed and 30 previously treated for breast cancer who underwent CPM a second time (because of later detection of BRCA mutation in 10 cases). The external lateral incision was the most frequent surgical technique. In all patients, immediate reconstruction was performed.
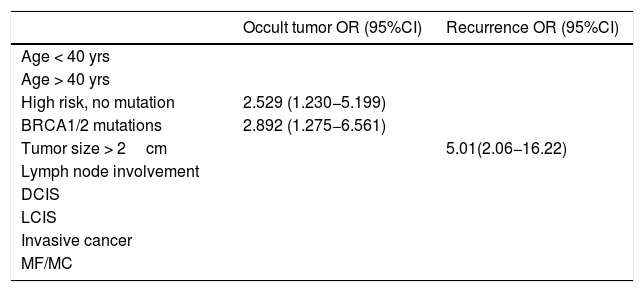
In CPM pieces, 4 in situ carcinoma, 3 invasive and 1 atypical hyperplasia were found. The incidence of occult contralateral cancer was 15.2%. Recurrence was observed in 5 patients a mean of 21.2 months after surgery. DFSD was 83.74 months and OS 84.33 months. Regression models identified BRCA1/2 mutation and high risk without mutation as significant occult tumor predictive factors while tumor size≥2cm was predictive of recurrence.
ConclusionsIn our series we found a 10.8% recurrence despite CPM and 7 patients (15.2%) would have developed a CBC in subsequent years.
La mastectomía contralateral profiláctica (CPM) reduce el riesgo de cáncer contralateral en al menos un 90%. Además, las portadoras de mutación BRCA tienen mayor riesgo de recurrencia ipsilateral y de un segundo tumor primario. El objetivo es evaluar el riesgo de cáncer contralateral y la recurrencia, y analizar factores predictivos en pacientes con cáncer de mama y mutaciones BRCA1/2 y no portadoras con alto riesgo de cáncer hereditario.
MétodosAnálisis observacional retrospectivo de 46 pacientes sometidas a mastectomía bilateral durante 2004–2018. Nueve pacientes BRCA1, 12 BRCA2 y 25 con alto riesgo sin mutación.
ResultadosDieciséis pacientes con diagnóstico de novo y 30 tratadas previamente por cáncer de mama a las que realizamos CPM de manera diferida (en 10 de ellas por detección de mutación en BRCA a posteriori); mediana de seguimiento 79 meses. La técnica quirúrgica más usada fue la incisión lateral externa. En todas las pacientes se realizó reconstrucción inmediata.
En las piezas de CPM se encontraron 4 tumores in situ, 3 invasivos y una hiperplasia atípica. La incidencia de cáncer contralateral oculto fue del 15,2%. Cinco pacientes presentaron recidiva 21,2 meses de media tras la intervención; SLE 83,74meses y SG 84,33meses. Los modelos de regresión identificaron mutación BRCA1/2 y alto riesgo sin mutación como factores predictivos significativos para tumor oculto, mientras que el tamaño tumoral≥2cm fue predictivo de recidiva.
ConclusionesEn nuestra serie 7pacientes (15,2%) habrían desarrollado un tumor contralateral en los años posteriores, y un 10,8% presentaron recurrencia a pesar de CPM.
Some 5%–10% of breast cancer patients carry at least one susceptibility gene, including TP53, PTEN, LKB1, MSH2/MLH1, BRCA1 and 2, the latter being due to mutations in most cases.1 According to a meta-analysis,2 women with a germline mutation in the BRCA1 or BRCA2 gene have a higher risk of developing breast cancer in their lifetimes (57% and 49%, respectively).2 Furthermore, they are at risk for contralateral breast cancer (CBC) that is 4.5 and 3.4 times higher3 as well as a higher risk of recurrence.4
In prospective observational studies, bilateral prophylactic mastectomy has been shown to decrease the incidence of breast cancer by 90% in patients with BRCA mutation. It also improves patient quality of life by reducing anxiety and fear of breast cancer. In patients who presented cancer, 2 factors were associated with a 50% decrease in the risk of CBC: bilateral salpingo-oophorectomy in BRCA2 (RR: 0.52; 95% CI: 0.37−0.74) and age over 40 years; also, an additional factor that reduced this risk was the use of adjuvant tamoxifen (RR: 0.57: 95% CI: 0.43−0.75).5 However, the benefits of contralateral prophylactic mastectomy in these patients have been more widely debated. A Cochrane systematic review established that contralateral prophylactic mastectomy (CPM) could prevent the increased risk of CBC without an impact on survival.6 Since 2014, there is new evidence that supports a benefit in overall survival (89% vs 71%, P<.001),7,8 regardless of whether salpingo-oophorectomy was performed, and especially in patients under the age of 40, stage 1 and 2, not triple negative and women not treated with chemotherapy.9 For this reason, it seems reasonable to perform bilateral mastectomy in patients with unilateral cancer carrying mutated BRCA genes.10
The objective of this study is to evaluate the risk of CBC and recurrence. We will also analyze predictive factors in patients with breast cancer carrying BRCA1/2 mutations and non-carriers with high risk of hereditary breast cancer.
MethodsOur retrospective observational analysis included patients with unilateral breast cancer carrying mutations in the BRCA1 and 2 genes who underwent bilateral mastectomy (as contralateral prophylactic mastectomy) from 2004 to 2018.
The criteria to perform the genetic test (BRCA1/2) were the following:
- -
Age at diagnosis ≤ 40 years
- -
Patients with multiple primary cancers
- -
Patients with known BRCA mutations in the family
- -
Patients with 2 first-degree relatives with breast cancer
- -
Patients with a first-degree relative with bilateral cancer and a second-degree relative with a history of breast cancer
- -
Patients with a first-degree relative with cancer <45 years old and a second-degree relative with a history of breast cancer.
Genetic counseling was first implemented in our hospital in 2013. Until then, prophylactic contralateral mastectomy had been offered to patients with breast cancer and a strong family history of breast cancer in the absence of a known mutation.
High-risk non-carrier or BRCA patients who opted for conservative surgery or unilateral mastectomy were excluded from the study. We also excluded patients with unilateral cancer who were not BRCA carriers or those without high family-related risk who underwent CPM for other reasons (tumor type, choice, etc) and patients with synchronous bilateral cancer, as the purpose of the study was to assess the risk of CBC.
A skin- and nipple-sparing mastectomy was performed with immediate reconstruction by placing a definitive silicone implant in all cases. Four patterns were used: mastectomy by external lateral incision, Wise pattern, Spira technique and external radial incision. The absence of disease at the base of the nipple-areola complex was confirmed by intraoperative biopsy.
Statistical analysisThe descriptive analysis of the variables under study: for qualitative variables, relative and absolute frequencies are provided; for quantitative variables, mean and standard deviation are given. In order to evaluate differences, the chi-square or Fisher’s tests were used in the case of qualitative variables, and the Mann Whitney U or Student’s t test for quantitative variables according to normality criteria. Logistic regression models were created to identify the predictors of occult cancer and recurrence. Other variables evaluated included: age, high risk due to family history, BRCA mutations, tumor size, lymph node involvement, and histology of the initial breast cancer, including multifocal or multicentric presentation. The log-rank test was used to assess overall survival, disease-free survival (DFS), and recurrence rate. DFS was calculated as the time from surgery to the onset of local or systemic recurrence during the study period.
A P value less than .05 was considered statistically significant. For the entire analysis, the SPSS 22.0 program for Windows (SPSS Ibérica, Madrid, Spain) was used.
This study complies with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee at our hospital.
ResultsDuring the study period, 46 patients were included. Nine of them (19.6%) were carriers of BRCA1 mutation, 12 BRCA2 (26.1%), and 25 patients (54.3%) were at high risk without mutation. A total of 16 patients (34.7%) had a de novo diagnosis, and 30 (65.2%) had been previously treated for breast cancer, so we performed CPM in a deferred manner (in 10 patients due to detection of mutation in BRCA genes a posteriori). The mean age of the patients was 47.4±9.2 years. The distribution of the patients is shown in Fig. 1.
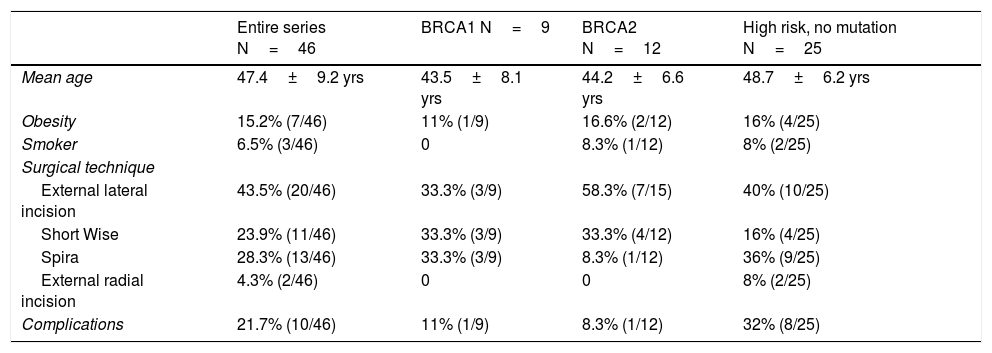
Bilateral mastectomy was performed with immediate reconstruction. The most frequently used technique was external lateral incision (43.5%, 20/46), followed by the Spira technique (28.3%, 13/46). Data for demographics and surgical technique are shown in Table 1. The overall complication rate was 21.7%, and no patient required reoperation.
Demographic data related to surgical technique.
| Entire series N=46 | BRCA1 N=9 | BRCA2 N=12 | High risk, no mutation N=25 | |
|---|---|---|---|---|
| Mean age | 47.4±9.2 yrs | 43.5±8.1 yrs | 44.2±6.6 yrs | 48.7±6.2 yrs |
| Obesity | 15.2% (7/46) | 11% (1/9) | 16.6% (2/12) | 16% (4/25) |
| Smoker | 6.5% (3/46) | 0 | 8.3% (1/12) | 8% (2/25) |
| Surgical technique | ||||
| External lateral incision | 43.5% (20/46) | 33.3% (3/9) | 58.3% (7/15) | 40% (10/25) |
| Short Wise | 23.9% (11/46) | 33.3% (3/9) | 33.3% (4/12) | 16% (4/25) |
| Spira | 28.3% (13/46) | 33.3% (3/9) | 8.3% (1/12) | 36% (9/25) |
| External radial incision | 4.3% (2/46) | 0 | 0 | 8% (2/25) |
| Complications | 21.7% (10/46) | 11% (1/9) | 8.3% (1/12) | 32% (8/25) |
The median follow-up time was 79 months (SD: 51.62).
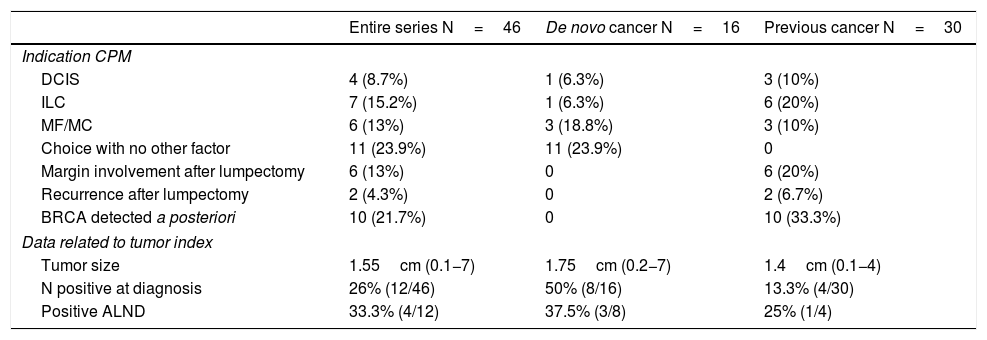
Out of the 16 patients with a newly diagnosed cancer, the indication for simultaneous CPM was determined by being a multicentric and/or multifocal tumor in 3 cases, ductal carcinoma in situ (DCIS) in one case, invasive lobular carcinoma (ILC) in one case, and patient choice in the remaining 11.
In the group of patients previously treated for a tumor, CPM was performed in a delayed manner: in 10 cases when the BRCA gene mutation was detected a posteriori, in 2 cases due to recurrence, in 6 due to involved margins after lumpectomy (and the patient’s preference to undergo BM instead of margin widening), in 6 due to ILC, 3 DCIS and 3 multicentric/multifocal tumors.
Mean tumor size was 1.65cm (0.1−7). In 12 patients, there was clinical lymph node involvement at diagnosis, for which axillary lymph node dissection (ALND) and selective biopsy of the sentinel lymph node on the contralateral side were performed. Only 4 were positive (4/12; 33%). Out of the remaining patients who underwent sentinel lymph node biopsy, this was only positive in 4 cases; ALND was performed, with a positive result in only 2 cases. The clinical and pathological data related to the tumor are shown in Table 2.
Clinical-pathological tumor data.
| Entire series N=46 | De novo cancer N=16 | Previous cancer N=30 | |
|---|---|---|---|
| Indication CPM | |||
| DCIS | 4 (8.7%) | 1 (6.3%) | 3 (10%) |
| ILC | 7 (15.2%) | 1 (6.3%) | 6 (20%) |
| MF/MC | 6 (13%) | 3 (18.8%) | 3 (10%) |
| Choice with no other factor | 11 (23.9%) | 11 (23.9%) | 0 |
| Margin involvement after lumpectomy | 6 (13%) | 0 | 6 (20%) |
| Recurrence after lumpectomy | 2 (4.3%) | 0 | 2 (6.7%) |
| BRCA detected a posteriori | 10 (21.7%) | 0 | 10 (33.3%) |
| Data related to tumor index | |||
| Tumor size | 1.55cm (0.1−7) | 1.75cm (0.2−7) | 1.4cm (0.1−4) |
| N positive at diagnosis | 26% (12/46) | 50% (8/16) | 13.3% (4/30) |
| Positive ALND | 33.3% (4/12) | 37.5% (3/8) | 25% (1/4) |
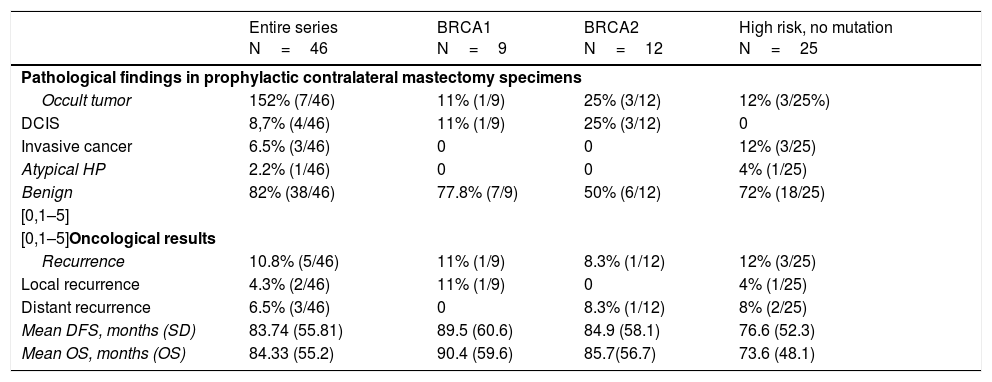
In the CPM specimens, 4 in situ tumors, 3 invasive tumors, and one atypical hyperplasia were found. The incidence of occult contralateral cancer was 15.2% (7/46) (P=.021).
Five patients had recurrence (2 local and 3 distant), with a mean of 21.2 months (range 1–59) after the intervention, and 3 died. The recurrence rate was 10.8% (5/46). Mean DFS time was 83.74 months, and mean overall survival was 84.33 months. The data are shown in Table 3.
Oncologic results.
| Entire series N=46 | BRCA1 N=9 | BRCA2 N=12 | High risk, no mutation N=25 | |
|---|---|---|---|---|
| Pathological findings in prophylactic contralateral mastectomy specimens | ||||
| Occult tumor | 152% (7/46) | 11% (1/9) | 25% (3/12) | 12% (3/25%) |
| DCIS | 8,7% (4/46) | 11% (1/9) | 25% (3/12) | 0 |
| Invasive cancer | 6.5% (3/46) | 0 | 0 | 12% (3/25) |
| Atypical HP | 2.2% (1/46) | 0 | 0 | 4% (1/25) |
| Benign | 82% (38/46) | 77.8% (7/9) | 50% (6/12) | 72% (18/25) |
| [0,1–5] | ||||
| [0,1–5]Oncological results | ||||
| Recurrence | 10.8% (5/46) | 11% (1/9) | 8.3% (1/12) | 12% (3/25) |
| Local recurrence | 4.3% (2/46) | 11% (1/9) | 0 | 4% (1/25) |
| Distant recurrence | 6.5% (3/46) | 0 | 8.3% (1/12) | 8% (2/25) |
| Mean DFS, months (SD) | 83.74 (55.81) | 89.5 (60.6) | 84.9 (58.1) | 76.6 (52.3) |
| Mean OS, months (OS) | 84.33 (55.2) | 90.4 (59.6) | 85.7(56.7) | 73.6 (48.1) |
Lastly, the univariate analysis was performed, and the regression models identified BRCA1/2 mutations and high risk without mutation as significant predictive factors for occult tumor (OR=2.892 [95% CI: 1.275−6.561] P=.003) (2.529 [1.230−5.199] P=.004), while tumor size ≥ 2 cm was predictive of recurrence (OR = 5.01 [95% CI: 2.06−16.22] P=.002) (Table 4).
Univariate regression models showing only results that are statistically significant.
| Occult tumor OR (95%CI) | Recurrence OR (95%CI) | |
|---|---|---|
| Age < 40 yrs | ||
| Age > 40 yrs | ||
| High risk, no mutation | 2.529 (1.230−5.199) | |
| BRCA1/2 mutations | 2.892 (1.275−6.561) | |
| Tumor size > 2cm | 5.01(2.06−16.22) | |
| Lymph node involvement | ||
| DCIS | ||
| LCIS | ||
| Invasive cancer | ||
| MF/MC |
It is a recognized fact that patients with a history of breast cancer have a 1.5–2 times higher risk of developing CBC than the general population,11 and it is even higher in carriers of mutations in the BRCA1 and BRCA2 genes of (4.5 and 3.4 times higher, respectively).3
The Metcalfe et al. study estimated a 10-year risk for CBC of 23.8% for BRCA1 and 18.7% for BRCA2,12 which is consistent with our results. Our study detected an occult tumor rate of 15.2%, and more than half were in situ tumors that would take time to show clinically.
Several factors have been shown to influence the risk of CBC in carriers of BRCA mutations with breast cancer, such as early age at diagnosis,12 with an increase in relative risk as the age at diagnosis increases.3 In our series, age less than 40 years was not significantly associated with an increased risk of presenting CBC.
The objective of treatment in patients with breast cancer is to minimize the risk of death from this tumor. However, in patients with mutations in BRCA genes or high risk of hereditary breast cancer without a known mutation, we must also minimize the incidence and mortality due to cancers that may later develop. Thus, intensive screening can help diagnose metachronous tumors in early stages but cannot prevent their development. Therefore, preventive strategies such as CPM should be considered, which in patients at risk reduces the risk of CBC by at least 90%.13 In our series, 7 patients (15.2%) would have developed a contralateral tumor in subsequent years.
On the other hand, among breast cancer patients treated with conservative surgery, BRCA mutation carriers have a higher risk of ipsilateral recurrence and of developing a second primary tumor compared to sporadic controls.11 In a multicenter study, Pierce et al.14 described a 10-year ipsilateral recurrence rate that is 2 times higher in BRCA patients, and the Metcalfe study12 quantified a risk of 11% for women with a BRCA1 mutation and 17% for BRCA2, which concurs with our results (recurrence rate 10.8%). However, no significant differences have been found in the development of recurrence between BRCA1/2 mutation carriers and high-risk non-carriers, which is probably due to the fact that the BRCA mutation carriers were compared with high-risk non-carriers and not with patients with sporadic breast cancer.
This study has the typical limitations of retrospective single-center study designs. The small number of patients in each subgroup translates into weak statistical power and wide CI. During the study period, there were substantial changes, such as the creation of a genetic counseling consultation that was able to detect more patients with risk mutations, in addition to implementing other prevention measures such as prophylactic salpingo-oophorectomy, and the occult cancer detection rate may possibly decrease in coming years.
In conclusion, the results of this study show that both BRCA1/2 mutation carriers and non-carriers at high risk of hereditary breast cancer have a high risk of developing CBC and recurrence.
Assessment should be considered in order to carry out preventive measures, such as CPM, regardless of the BRCA1/2 mutation status in high-risk patients and mainly determined by family history.
Conflict of interestsThere are conflicts of interest to declare.
The authors would like to thank the Breast Unit at the Hospital Clínico Universitario Lozano Blesa in Zaragoza, Spain, including surgeons, nursing staff and patients
Please cite this article as: Allué Cabañuz M, Domingo Bretón M, Chóliz Ezquerro J, Arribas del Amo MD, Güemes Sánchez AT. Cáncer de mama contralateral y recurrencia en portadoras BRCA1/2 y no portadoras con alto riesgo de cáncer de mama hereditario tras mastectomía bilateral. Cir Esp. 2020;98:612–617.