Obesity surgery is the best treatment for extreme obesity, with demonstrated long-term positive outcomes. The potential cost-savings generated by the improvement of comorbidities after surgery can justify the allocation of more resources in the surgical treatment of obesity.
MethodsThis was an observational, descriptive, longitudinal and retrospective study. Eligible patients underwent Roux-en-Y gastric bypass surgery at the Hospital Universitario Central de Asturias between 2003 and 2012. The established minimum follow-up period was two years. We calculated the individualized cost per patient treated (bottom-up) as well as per Diagnosis-Related Group (DRG) codes (top-down).
ResultsOur study included 307 patients. The average cost per hospitalization calculated by DRG codes was €6545.90, and the average cost per patient was €10572.20. DRG 288 represented 91% of the series, with a value of €4631. The number of medications also decreased during this period, from 2.86 to 0.78 per medically treated patient, representing a cost reduction of €4433 per patient with all the obesity-related comorbidities analyzed.
ConclusionsTwo years after Roux-en-Y gastric bypass conducted at Hospital Universitario Central de Asturias, the savings in drug costs for patients with multiple pathologies would compensate the inherent costs of the surgical treatment itself. Our results showed that DRG-related costs were insufficient to make a correct economic evaluation, so we recommend an individualized cost calculating method.
La cirugía bariátrica es el mejor tratamiento de la obesidad mórbida a largo plazo. El ahorro generado por la mejoría de las comorbilidades podría justificar el empleo de más recursos sanitarios.
MétodosEstudio observacional, descriptivo, longitudinal y retrospectivo, de pacientes a los que se les realizó un bypass gástrico, en el Hospital Universitario Central de Asturias entre 2003 y 2012. El seguimiento mínimo se estableció en dos años. Calculamos de manera individualizada el coste para cada uno de los pacientes intervenidos (bottom-up), así como según el grupo relacionado por el diagnóstico (GRD) (top-down).
ResultadosDe los 307 pacientes del estudio, el coste medio del ingreso calculado por GRD fue de 6.545,9€ y el calculado por paciente de 10.572,2€. El GRD 288 representa al 91% de la serie con un valor de 4.631€. El cálculo estimativo del ahorro que supuso en nuestro entorno sanitario la disminución del número de fármacos de 2,86 a 0,78 por paciente medicado, representó 4.433€ por paciente intervenido si padecía todas las comorbilidades analizadas.
ConclusionesEl bypass gástrico en el Hospital Universitario Central de Asturias a los dos años de la cirugía, en pacientes con pluripatología consiguió un ahorro solo en fármacos que podría compensar los gastos inherentes al tratamiento quirúrgico. El coste por proceso mediante GRD se mostró insuficiente a la hora de hacer una correcta evaluación económica, por lo que recomendamos un método de evaluación de coste por paciente.
Obesity and its comorbidities are a major public health and economic problem. In Spain, the prevalence of obesity in adults is over 20%, while 40% are overweight.1 The individual consequences are seen in both life expectancy and quality of life.
The treatment of obesity is multidisciplinary, and, after medical, pharmacological and behavioral treatments in established morbid obesity,2,3 the treatment that is most effective is bariatric surgery. Gastric bypass (GBP) is the bariatric procedure that has stood the test of time over other techiques,4,5 and it has proven to be the most cost-effective.6,7
The public healthcare demand for bariatric surgery is increasing, not only due to the obesity epidemic that we are experiencing, but also because patients and professionals are encouraged to choose this therapeutic option given the good clinical results and the low rate of complications. The practice of this surgery is currently limited, however, due to the growth of waiting lists,8 the supposed cost of the procedure, etc.
The cost of the hospitalization process can be calculated using two very different models: the traditional top-down model, which is based on the relative weight of the groups according to the diagnosis (DRG); and the bottom-up model, which is calculated per patient and per episode.9 The cost of bariatric surgery studied to date in our country has been based on traditional methods of economic evaluation, which are good10 but presumably insufficient.
Our intention is to evaluate the cost of the hospitalization process and the savings generated by the resolution of comorbidities.
MethodsPatientsWe have carried out an observational, descriptive, longitudinal and retrospective study on a prospective database of patients undergoing bariatric surgery between 2003 and 2012 who underwent GBP. This is the main technique performed at the Hospital Universitario Central de Asturias (HUCA), a tertiary hospital and the only public facility in Asturias where bariatric surgery is performed. It covers a population of around one million inhabitants. We discarded the first 30 patients with open and laparoscopic surgery, considering these cases a learning curve. The Research Ethics Committee of the Principality of Asturias authorized this Project (no. 10/16). The sample size was not calculated as it was the complete series. The article has been written following the STROBE11 model.
Waiting List Inclusion ProtocolAll the patients were studied, treated and referred to our consultation through their endocrinologist, following established indication criteria.12 In patients with a BMI greater than 60kg/m2, or greater than 50kg/m2 and with significant comorbidities, placement of an intragastric balloon (IGB) was indicated prior to surgery.
Comorbidities Related to ObesityAlthough various variables have been evaluated in the study, here we will consider those with economic impact during patient progress: arterial hypertension (HTN), type 2 diabetes mellitus (T2D), dyslipidemia (DL), obstructive sleep apnea syndrome (OSAS), osteoarthritis and depression. Although it may be debatable at the present time, cholecystectomy was performed and evaluated separately due to the presence of cholelithiasis or inherent to the distal gastric bypass due to BMI>55kg/m2.
Surgical ProcedureAll patients underwent scheduled surgery in the same operating room of the HUCA after outpatient consultation. There were two main surgeons, both present in most of the procedures, but at least one was present in all of them.
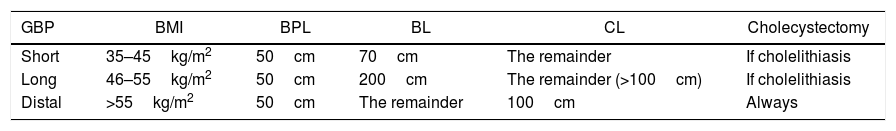
The GBP type was standard in the gastric part (restrictive), on the lesser curvature and adapted for a capacity of around 30mL, with a 25mm circular gastrojejunal anastomosis. The intestinal (malabsorptive) part varied depending on BMI (Table 1).
Length of Loops According to BMI.
| GBP | BMI | BPL | BL | CL | Cholecystectomy |
|---|---|---|---|---|---|
| Short | 35–45kg/m2 | 50cm | 70cm | The remainder | If cholelithiasis |
| Long | 46–55kg/m2 | 50cm | 200cm | The remainder (>100cm) | If cholelithiasis |
| Distal | >55kg/m2 | 50cm | The remainder | 100cm | Always |
BL: bowel loop; BPL: biliopancreatic loop; CL: common loop; GBP: gastric bypass; BMI: body mass index.
Laparoscopic surgery has been gradually implemented since 2007, when more than 100 open GBP had already been performed, the first cases being more selected for their more favorable BMI. Upon discharge, a clinical and surgical data collection form was completed.
Exclusion CriteriaWe excluded from the study patients who did not give their consent, active smokers, those with revision surgeries, and patients with psychiatric pathologies that prevented them from understanding or complying with the dietary and drug modifications that surgery entailed.
Follow-upFollow-up office visits were scheduled one month, 6 months, one year and two years after surgery. We calculated the percentage of excess weight lost (%EWL), whether there was a reduction or not of drug needs, and whether new medications were needed to control comorbidities. In the evolution of comorbidities, we distinguished between: worse, when the clinical situation worsened or more medication was required; same, when there was no change from the starting situation; better, if there was a reduction in the number of drugs, dose or frequency, as well as a decrease in the CPAP requirements; and, resolved, in the absence of medication or devices, and recovery of normal clinical or analytical values.
Economic EvaluationThe cost of the analyzed drugs was obtained from the information catalog of products included in the pharmaceutical provisions of the Spanish Ministry of Health.13 Due to the great variability and rotation of medications in the different reviews, we opted to use the costs obtained from publications in our country.14–18
To calculate the costs of the procedure, we used two models, the first using analytical accounting by DRG, for which the Admissions Service was asked to code all the patients based on their history number and dates of admission, surgery and discharge, as well as re-admission when it occurred. They returned the DRG in which each of the admission episodes were grouped, together with their cost. The second was an individualized calculation (much more complex but more precise) resulting from the breakdown of each of the phases of admission. On the one hand, we calculated the cost of admission by days of hospital stay, to which we added the cost of using the surgical area (provided by the Hospital Management Control Service); we also added the time spent by surgeons and anesthetists, and the material used. Both the studies and the pre- and postoperative consultations were collected and added to the final cost of the process, in the same way as complications or readmissions.
Statistical AnalysisThe statistical analysis was conducted using freely distributed R statistical software (R Foundation for Statistical Computing, Vienna, Austria).19 We carried out a descriptive statistical analysis, expressing the continuous variables as means and standard deviation or median and range. Categorical variables are expressed as frequencies and percentages.
The comparison by groups was performed using the Welch test when the variables were symmetric and the non-parametric Kruskal–Wallis test when the variables were asymmetric. Categorical variables were compared using the chi-squared test.
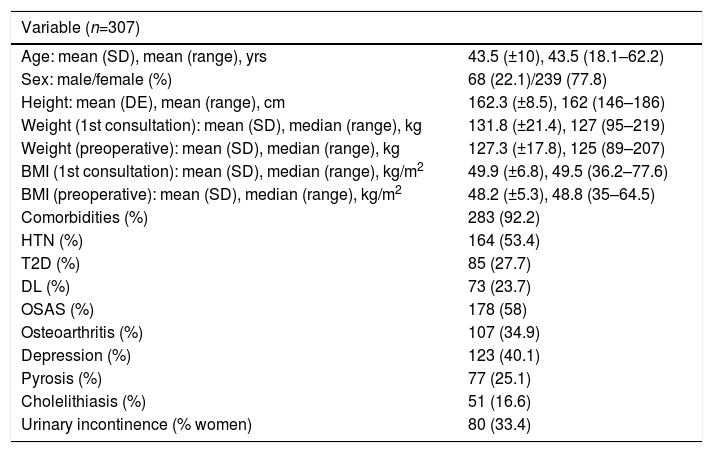
ResultsOut of the 384 patients operated on at HUCA from the start of the bariatric surgery program until December 2012, 77 were excluded from the study: 17 because the GBP was not primary, and the remaining 60 because they were considered part of the learning curve. There were therefore 307 valid patients for the study, whose general characteristics are shown in Table 2.
General Characteristics of the Series.
| Variable (n=307) | |
|---|---|
| Age: mean (SD), mean (range), yrs | 43.5 (±10), 43.5 (18.1–62.2) |
| Sex: male/female (%) | 68 (22.1)/239 (77.8) |
| Height: mean (DE), mean (range), cm | 162.3 (±8.5), 162 (146–186) |
| Weight (1st consultation): mean (SD), median (range), kg | 131.8 (±21.4), 127 (95–219) |
| Weight (preoperative): mean (SD), median (range), kg | 127.3 (±17.8), 125 (89–207) |
| BMI (1st consultation): mean (SD), median (range), kg/m2 | 49.9 (±6.8), 49.5 (36.2–77.6) |
| BMI (preoperative): mean (SD), median (range), kg/m2 | 48.2 (±5.3), 48.8 (35–64.5) |
| Comorbidities (%) | 283 (92.2) |
| HTN (%) | 164 (53.4) |
| T2D (%) | 85 (27.7) |
| DL (%) | 73 (23.7) |
| OSAS (%) | 178 (58) |
| Osteoarthritis (%) | 107 (34.9) |
| Depression (%) | 123 (40.1) |
| Pyrosis (%) | 77 (25.1) |
| Cholelithiasis (%) | 51 (16.6) |
| Urinary incontinence (% women) | 80 (33.4) |
SD: standard deviation; DL: dyslipidemia; T2D: type 2 diabetes mellitus; HTN: hypertension; BMI: body mass index; OSAS: obstructive sleep apnea syndrome.
The waiting-list time after the patient was assessed in our consultation until the procedure was 12.2 (±3.8) months (range 1–27.8 months). The placement of an IGB prior to surgery was indicated in 46/307 patients (15%). The mean weight loss compared to the day prior to placement was 24.6 (±10) kg.
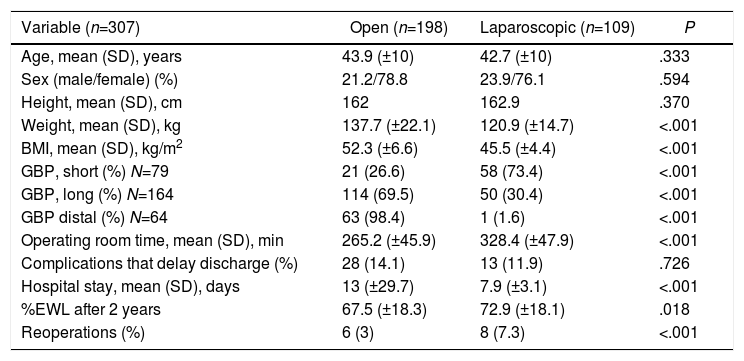
GBP was performed on all patients, and the length of the loops varied according to BMI (Table 1). The initial approach was open in 189/307 (61.5%) cases, to which 9 conversions must be added (7.6%). Table 3 shows the characteristics of the series according to the approach used.
Characteristics of the Population According to Surgical Approach.
| Variable (n=307) | Open (n=198) | Laparoscopic (n=109) | P |
|---|---|---|---|
| Age, mean (SD), years | 43.9 (±10) | 42.7 (±10) | .333 |
| Sex (male/female) (%) | 21.2/78.8 | 23.9/76.1 | .594 |
| Height, mean (SD), cm | 162 | 162.9 | .370 |
| Weight, mean (SD), kg | 137.7 (±22.1) | 120.9 (±14.7) | <.001 |
| BMI, mean (SD), kg/m2 | 52.3 (±6.6) | 45.5 (±4.4) | <.001 |
| GBP, short (%) N=79 | 21 (26.6) | 58 (73.4) | <.001 |
| GBP, long (%) N=164 | 114 (69.5) | 50 (30.4) | <.001 |
| GBP distal (%) N=64 | 63 (98.4) | 1 (1.6) | <.001 |
| Operating room time, mean (SD), min | 265.2 (±45.9) | 328.4 (±47.9) | <.001 |
| Complications that delay discharge (%) | 28 (14.1) | 13 (11.9) | .726 |
| Hospital stay, mean (SD), days | 13 (±29.7) | 7.9 (±3.1) | <.001 |
| %EWL after 2 years | 67.5 (±18.3) | 72.9 (±18.1) | .018 |
| Reoperations (%) | 6 (3) | 8 (7.3) | <.001 |
GBP: gastric bypass; SD: standard deviation; BMI: body mass index; %EWL: percent excess weight lost.
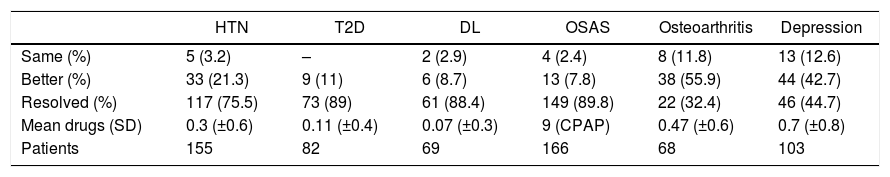
The two-year follow-up was completed in 287 patients (93.4%). The situation of the comorbidities after two years is shown in Table 4.
Evolution of Comorbidities Two Years After Surgery.
| HTN | T2D | DL | OSAS | Osteoarthritis | Depression | |
|---|---|---|---|---|---|---|
| Same (%) | 5 (3.2) | – | 2 (2.9) | 4 (2.4) | 8 (11.8) | 13 (12.6) |
| Better (%) | 33 (21.3) | 9 (11) | 6 (8.7) | 13 (7.8) | 38 (55.9) | 44 (42.7) |
| Resolved (%) | 117 (75.5) | 73 (89) | 61 (88.4) | 149 (89.8) | 22 (32.4) | 46 (44.7) |
| Mean drugs (SD) | 0.3 (±0.6) | 0.11 (±0.4) | 0.07 (±0.3) | 9 (CPAP) | 0.47 (±0.6) | 0.7 (±0.8) |
| Patients | 155 | 82 | 69 | 166 | 68 | 103 |
CPAP: continuous positive airway pressure; SD: standard deviation; DL: dyslipidemia; T2D: type 2 diabetes mellitus; HTN: hypertension; OSAS: obstructive sleep apnea syndrome.
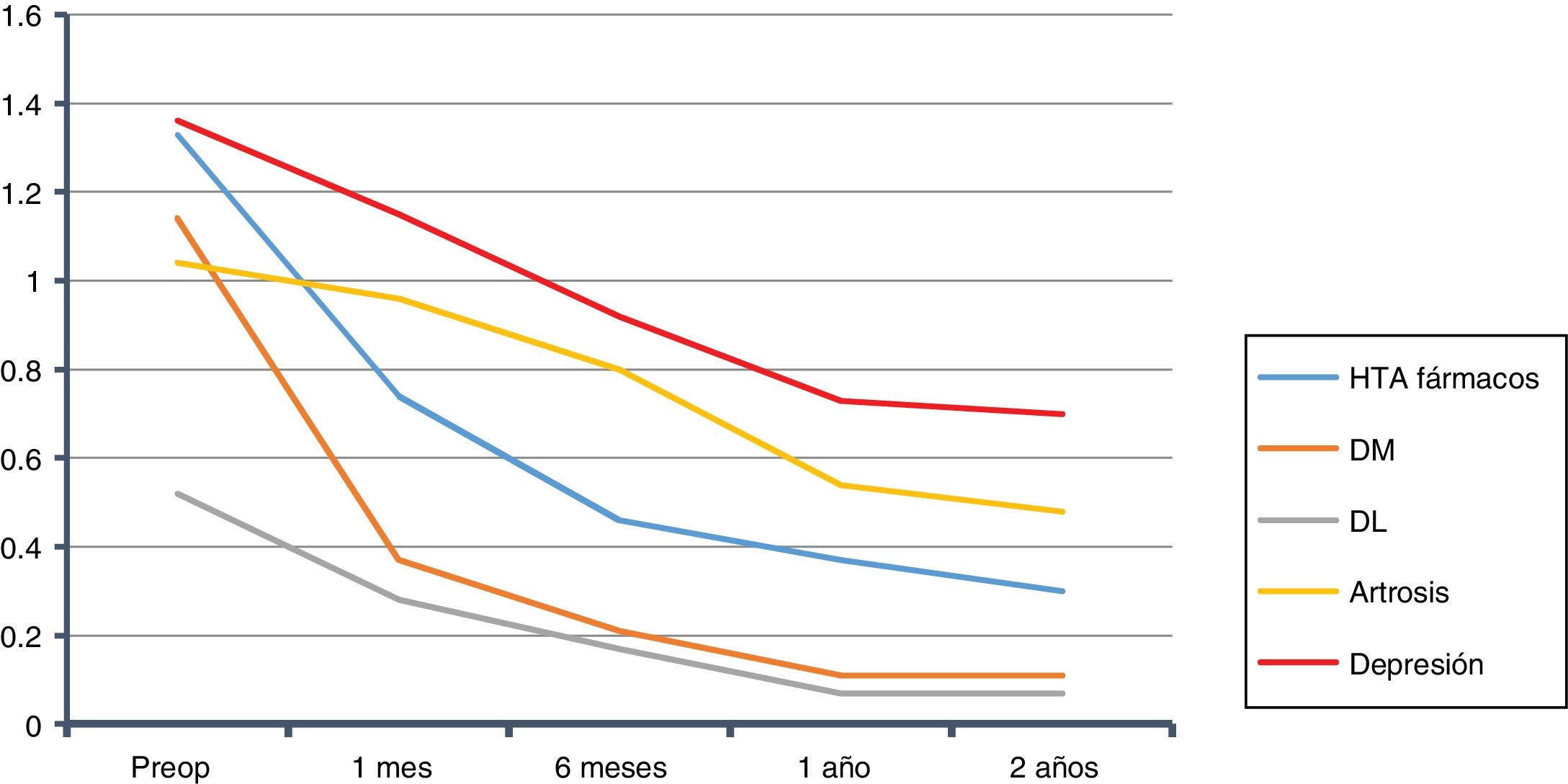
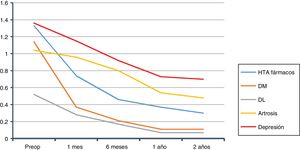
The overall evolution of the decrease in drug consumption is shown in Fig. 1. The final total of drugs was 164; in the 210 patients who had been medicated at the beginning of the study, the ratio of drugs per patient was 0.78, compared to 2.86 before the intervention (602/210).
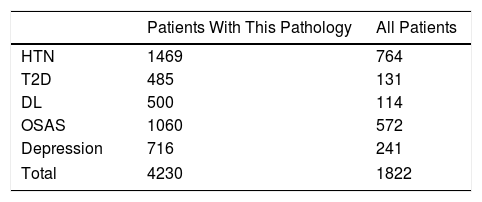
We calculated the savings for each of the pathologies over the course of two years, both for the complete sample and for each group of patients with that specific morbidity. In the case of HTN14: €764 per patient in the sample, and €1469 per patient with HTN. For T2DM15: €131 per patient in the sample, and €485 per patient with T2DM. In DL16: €114 per patient in the sample, and €500 per patient with DL. For OSAS17: €572 per patient in the sample, and €1060 per patient with OSAS. Those with depression18: €241 per patient in the sample, and €716 per patient with depression. In total, in two years we observed a drug savings of €1822 per patient treated surgically in our series, and a savings of €4230 per patient when they presented the five diseases analyzed (Table 5). Cholecystectomy was performed according to protocol in 97 patients. Given the annual incidence of complications, the number of patients who will develop cholelithiasis and those who will become symptomatic during follow-up,20–22 if these procedures had not been done, 20 patients would require re-hospitalization for cholecystectomy. Also, as they were all laparoscopic approaches without complications (DRG 494), which in 2013 at HUCA had a cost of €3126.62, for a total of €62532, this, divided by the sample, gives us a savings of €203 per patient in the sample.23
The cost per procedure was collected by DRG (version 27.0 for 2013), with 269 patients in DRG 288 (€4631.60), which represents an adjusted percentage of 91%, 25 patients in 565 (€13558), 2 in 877 (€124311.30) and 11 not coded, with an average cost of €6545.90.
The individualized cost requires a complex breakdown23 that includes the costs of personnel, pharmacy, supplies, pathology, laboratory, nursing, operating room, recovery, imaging diagnosis and food. In short, the average individualized cost of admission was €10572.20.
The comparison of both methods of calculating costs gives us a P<.001.
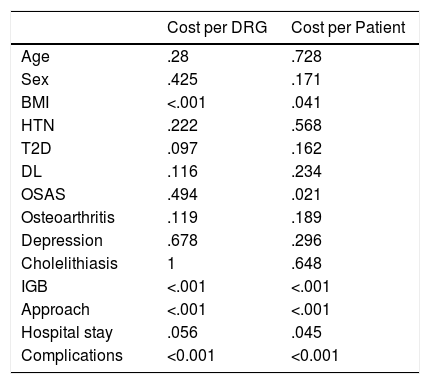
Once the clinical and economic parameters were collected, we proceeded to carry out the analysis of the results of our patients following the cost calculation model (Table 6). The cost per DRG was more expensive in patients with higher BMI and in those who presented complications or required an IGB, and cheaper in those who underwent laparoscopy.
Level of Significance (P) of the Statistical Tests Used.
| Cost per DRG | Cost per Patient | |
|---|---|---|
| Age | .28 | .728 |
| Sex | .425 | .171 |
| BMI | <.001 | .041 |
| HTN | .222 | .568 |
| T2D | .097 | .162 |
| DL | .116 | .234 |
| OSAS | .494 | .021 |
| Osteoarthritis | .119 | .189 |
| Depression | .678 | .296 |
| Cholelithiasis | 1 | .648 |
| IGB | <.001 | <.001 |
| Approach | <.001 | <.001 |
| Hospital stay | .056 | .045 |
| Complications | <0.001 | <0.001 |
IGB: intragastric balloon; DL: dyslipidemia; T2D: type 2 diabetes mellitus; DRG: diagnosis-related group; HTN: hypertension; BMI: body mass index; OSAS: obstructive sleep apnea syndrome.
The cost per patient shows differences according to BMI: cheaper at lower BMI, with shorter hospital stays. Among the most expensive were those with complications, those with a IGB and those with a laparoscopic approach.
DiscussionThe results of our series show us a population with characteristics similar to previous series.2 From a surgical standpoint, we had a 7.6% conversion rate, which is far from what is recommended (currently below 3%24) and a high percentage of reoperations, especially in the laparoscopic group. The differences found between the two approaches are a consequence of our selection of patients with lower weight for laparoscopy. In the laparoscopic group, the operating room time was significantly longer, but the hospital stay was shorter.
The resolution of comorbidities in the case of HTN was within expectations,25,26 which we calculated based on previous studies, putting the annual cost at €894.14 In the case of DM, our clinical results were better than expected27; the cost that we took as reference at the time of the study was €265 per year of medication,15 since this represents about 15% of the health expenditure of patients with T2D with an estimated cost of around €1770/year, although this figure today would probably be underestimated.28,29 The resolution of DL had an annual cost of €282,16 and OSAS is estimated at €675 per year, with a cost per session of €1.85.17 Osteoarthritis and depression are the pathologies that respond the worst. We did not make an economic assessment of the former due to the complexity, but for depression we assumed a savings per year of €1000.18
In the US, the cost of laparoscopic GBP varies from $1740030 to $25000.31,32 In Spain, it stands at €7468 based on DRG,10 which at our hospital was €4631; by individualized cost, it is €5483 for laparoscopic GBP,33 and in our evaluation somewhat above ten thousand euros.
Thus, with regard to the cost of hospitalization, the classical method for accounting based on DRG has been shown to be limited, as it is not influenced by such important data as days of hospital stay.34 The bottom-up method has been shown to behave in a more reliable and individualized manner.
To study the savings that this surgery could entail over the course of two years, we have focused on the consumption of drugs and devices in the most prevalent morbidities due to the characteristics of the study. Nonetheless, a large number of uncontrollable costs also come into play.
We started at 2.86drugs/medicated patient, and 2 years later we had 0.78. This is a reduction of around 70%, which is similar to previous studies.35,36 In other studies, this reduction was even greater37: from 2.4 drugs on average before surgery to 0.2 per year.
After two years, we observed a savings in drugs of €1822 per surgical patient in our series, and €4230 in patients who presented the five pathologies analyzed. As in previous studies,38–41 it is accepted that bariatric surgery, with the GBP of choice,6,7 has an economic benefit in terms of fewer drugs, although it is not clear whether this is a cost-savings.42 What we can confirm is that our calculation underestimates the actual savings, since this reduction in drugs is not limited exclusively to two years, nor only to medication. This is especially evident in patients with T2D, who require many healthcare services; this group would be sensitive to prioritization,39,43 as delayed surgery would delay clinical benefits.44 At this point, we can add the €203 generated by cholecystectomy to our calculation, giving us a final total (€4433) that is close to the cost that HUCA defines for this type of procedure (€4631) with the economic evaluation system it utilizes (DRG 288).
As could be assumed, the results indicate that the patients who consume the most resources are those who present complications and those who underwent IGB placement, to the extent that both compute an increase in cost (per DRG or individualized). Those who are estimated to consume the least by DRG are laparoscopic procedures because these do not take into account the material used or the operating room time. In the individualized calculation, patients that consume fewer resources are those with lower BMI, who present fewer complications and those requiring shorter hospital stays. The limitations of the DRG calculation are evident.
Lastly, let us remember that obese patients consume more resources per se, regardless of the procedure to be performed or reason for admission.45,46 They are patients who require a large number of services, both outpatient, hospital, and pharmacological. At the current rate, they can make the healthcare system unsustainable, representing an important challenge of our time that requires the collaboration and effort of everyone.47 The sooner we reverse their situation, the sooner we will return them to a healthy state, in which the costs are lower, and we will also improve their quantity and quality of life.
This cost analysis is limited for several reasons, as we have not evaluated the costs of unemployment, absenteeism, and the costs derived from treating sequelae of bariatric surgery. The drug cost was an estimation because of the large number of commercial presentations and prices for the same active ingredient. Likewise, the resolution of comorbidities did not follow the standardized parameters as currently recommended.48 This is a retrospective study with a short-term follow-up, at a single center, which limits extrapolation to others. In contrast, this is also a strength due to the complexity to make an individualized calculation of costs per admission at a hospital that does not use this type of cost assessment.
In conclusion, at our hospital, GBP in patients with multiple pathologies achieves savings in drugs alone that could offset the inherent costs of surgical treatment within two years of surgery. The cost per process using DRG is insufficient to make a correct economic evaluation, so we recommend a method that evaluates costs per patient.
Authorship/collaboratorsJosé Luis Rodicio: study concept and design, data collection, data analysis and interpretation, composition of the article, approval of the final version
Josefina Alonso, María Moreno, Amaya Rizzo, Estrella Turienzo and Lourdes Sanz: data analysis and interpretation, critical review, approval of the final version
José Ignacio Rodríguez and Juan González: study concept and design, data analysis and interpretation, critical review, approval of the final version
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Rodicio Miravalles JL, Alonso Fernández J, Moreno Gijón M, Rizzo Ramos A, Turienzo Santos E, Sanz Álvarez L, et al. Evaluación económica del tratamiento quirúrgico de la obesidad. Cir Esp. 2020;98:381–388.