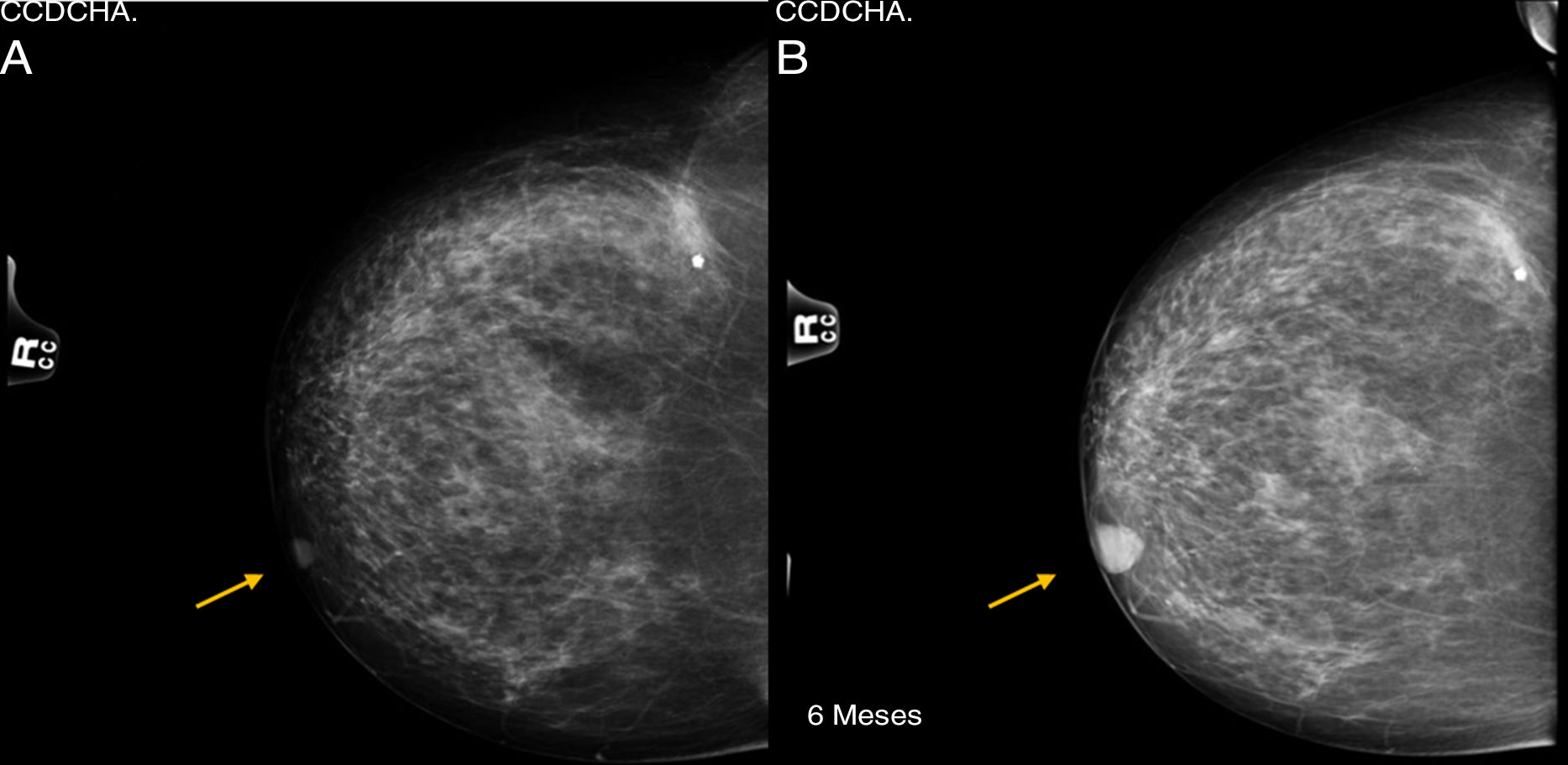
Tumors derived from the sweat glands are very uncommon neoplasms. Among them, clear-cell hidradenocarcinoma (malignant nodular hidradenoma), originating in eccrine sweat glands, is extremely rare. We present the case of a 76-year-old female patient, with a history of sebaceous cyst in the left breast that had been previously removed, who came to the consultation after palpating a mass in the right breast. During examination, a well-defined, mobile, superficial retroareolar mass was observed with negative axillary exploration, giving the impression of a benign breast pathology. Mammography and ultrasound studies showed no radiological translation of the lesion, which was interpreted as BIRADS-1. Six months later, after an increase in the nodule on palpation, both studies were repeated, identifying growth from 12 to 19mm of a lesion that was initially interpreted as a shadow of the nipple. Given the benign radiological appearance, as well as the history of sebaceous cyst, the lesion was classified as BIRADS-2 (Fig. 1).
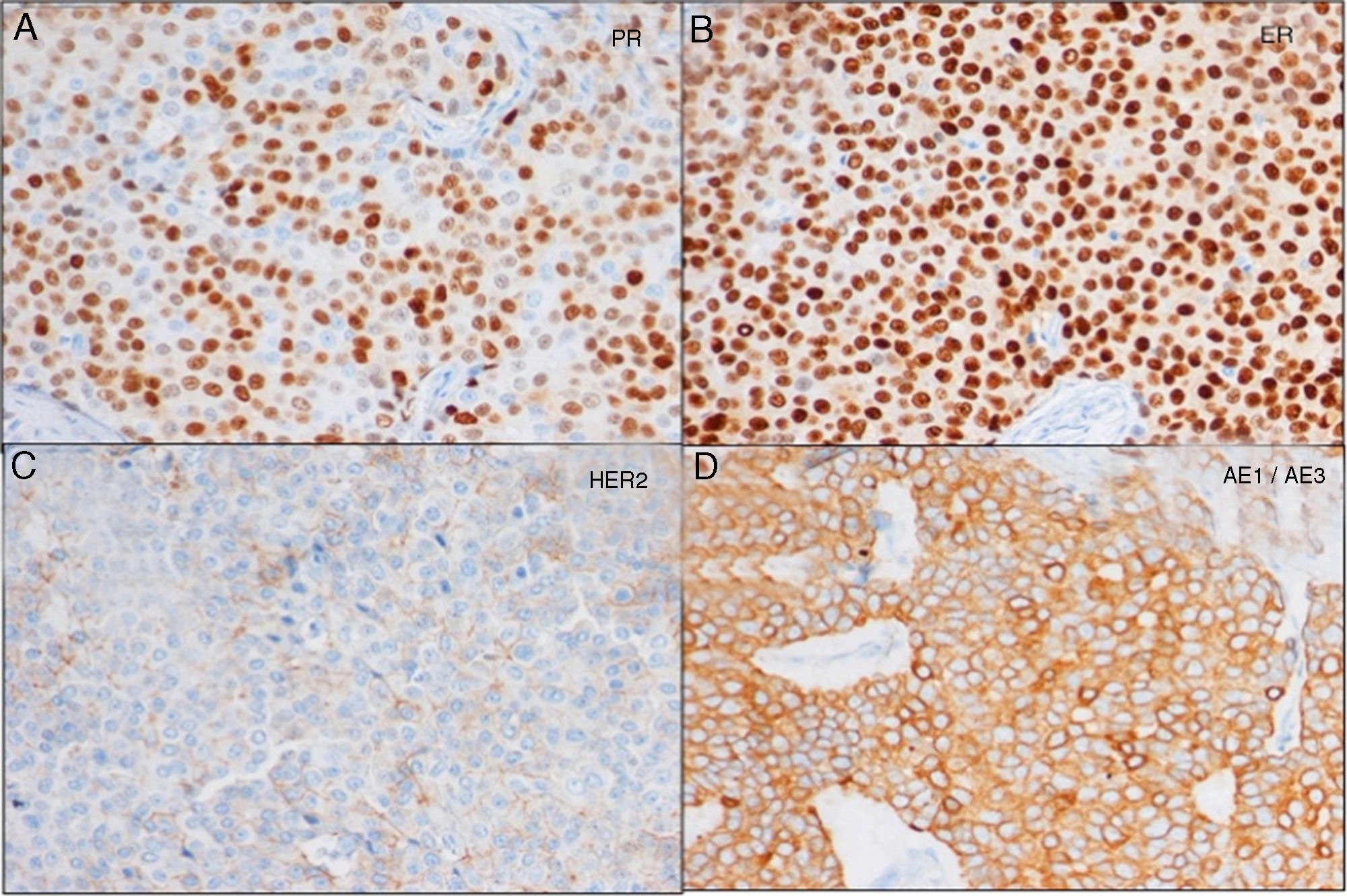
Lumpectomy was performed, including a cutaneous wedge. The histopathological study reported the lesion was a clear-cell hidradenocarcinoma, encapsulated and in contact with the stained surgical margin. The immunohistochemical study showed intense positivity for cytokeratins and estrogen (+++ in 100%) and progesterone (+++ in 70%) receptors, indeterminate HercepTest® 2+ (heterogeneous positivity, negative FISH), diffusely positive CQ19 and MIB1 10% (Fig. 2). When the case was discussed in the multidisciplinary committee, a simple mastectomy was indicated (as it was impossible to widen the margins while retaining esthetics) with selective biopsy of the sentinel lymph node (2 negative nodes). The mastectomy piece showed no residual tumor. An extension study showed no signs of distant involvement. It was decided not to administer adjuvant treatment and instead carry out a periodic clinical follow-up. Three years after diagnosis, the patient is currently disease free.
Clear-cell hidradenocarcinomas are aggressive tumors that account for less than 6% of malignant eccrine gland tumors (less than 1% of all cutaneous neoplasms). There have been only 50 cases reported in the literature, generally located on the face, scalp, torso and extremities. They usually appear de novo on healthy skin, although they sometimes transform from the benign form (clear-cell hidradenoma).1 When the location is the breast, the lesions are usually located in the areolar region, although they can appear in deeper glandular tissue.2 The presentation predominates slightly in the fifth decade of life and the female sex.3 Clinically, the presentation is that of a firm and painless subcutaneous nodule that may secrete serosanguinous fluid. Microscopically they are constituted by epithelial tumor cells arranged in lobes with a fibrovascular stroma and PAS + areas. They present central necrosis, atypical mitoses, anaplastic cells and signs of vascular permeation, which are characteristics that define malignancy. Immunohistochemistry studies are usually positive for cytokeratins, CEA, EMA, GCDFP-15 and p63, but negative for vimentin. Screening for HER2 overexpression and estrogen receptors4 should be considered in the tumor sample due to its potential usefulness. Due to the cutaneous location, the differential diagnosis should include basal cell and squamous cell carcinomas (epidemiologically more frequent), benign hidradenoma and, because of its clear cells, with metastatic carcinoma of the kidney, vagina, endometrium or salivary gland. The lesion presents slow growth for years,5 which means it is not aggressive. However, it recurs locally in 50% of cases, and metastasizes in up to 60% within 2 years (regional lymph nodes, peritoneal or mediastinal lymph nodes, lung, bone, brain and liver, in order of frequency). The prognosis is bad given its ability to recur and metastasize, presenting a 5-year overall survival of less than 30%. The series of 7 cases presented by Souvatzidis et al. in 2007 recorded an average survival of 25.6 months after diagnosis.6
Early diagnosis and treatment are the two variables that most influence long-term prognosis. Survival depends on the size and the systemic dissemination of the tumor. The only treatment that has been shown to positively influence survival is extensive surgical resection of the tumor (margins between 3 and 5cm are recommended to ensure negativity). Diagnostic surgery is usually performed, requiring a second intervention to extend margins and study the lymph nodes once the tumor type has been determined.7 The study of the sentinel lymph node is recommended,8 requiring lymphadenectomy associated with radiotherapy in case of involvement. The efficacy of prophylactic lymphadenectomy has not been proven.
Adjuvant treatment with chemotherapy and radiotherapy has not been shown to influence local control of the disease or survival, but should only be considered in cases of large tumor size, high-risk histopathological characteristics or metastasis at the time of diagnosis.9 The treatment of recurrences is based on surgical reoperation, with or without associated radiotherapy and evaluating the possibility of hormone therapy or directed treatment against HER2 (Herceptin®) depending on the tumor immunohistochemistry, since these treatments seem to produce stability of the disease when there are recurrences or metastatic disease.10 In the case presented, the preoperative suspicion was a benign tumor, and the diagnosis was incidental. This corroborates the diagnostic ambiguity previously described by other authors. Given the limited incidence of this tumor, there is not sufficient scientific evidence in the literature to make recommendations for postoperative management and follow-up.
Please cite this article as: López Rojo I, Gómez Ramirez J, Tejedor Togores P, Rivas Fidalgo S, Díaz Miguel M. Hidroadenocarcinoma de células claras de localización mamaria. Cir Esp. 2018;96:308–310.
Presented at the 33rd Conference of the Spanish Society of Senology and Breast Pathology in Vigo, Spain, 16–18 October, 2014.