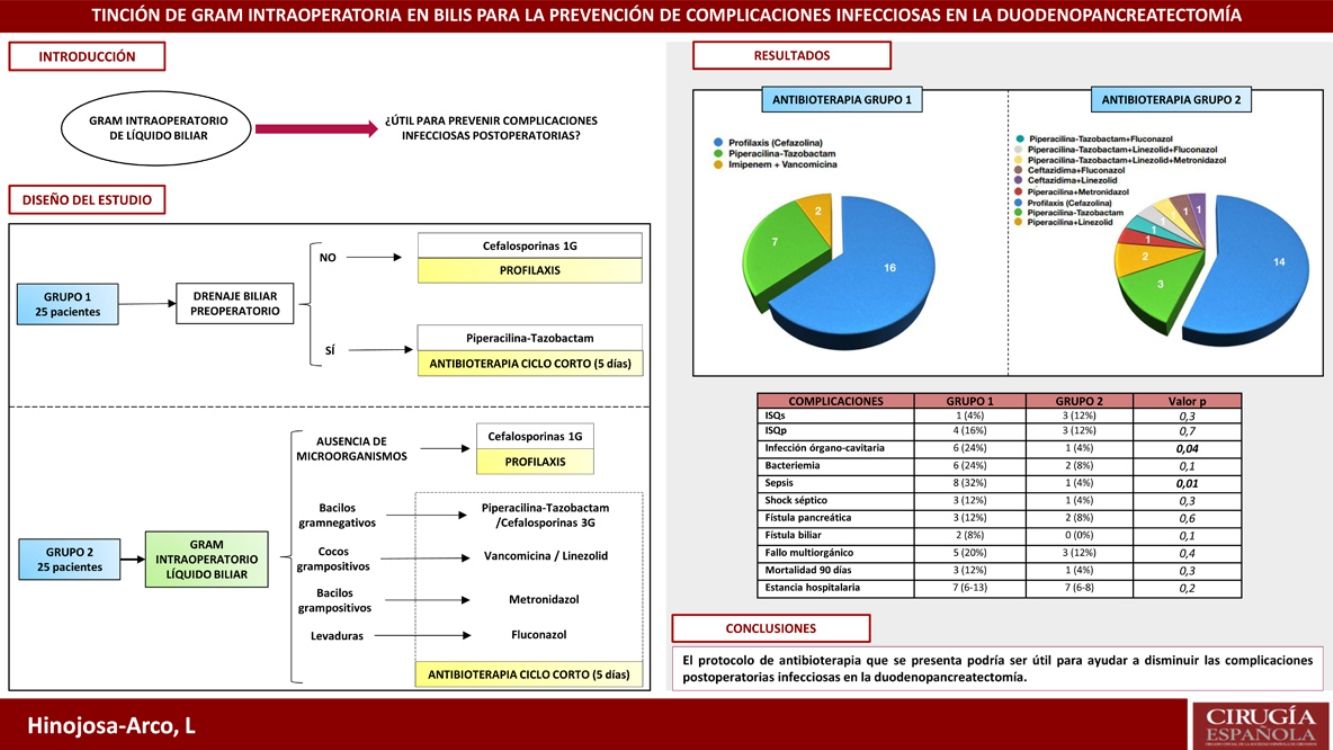
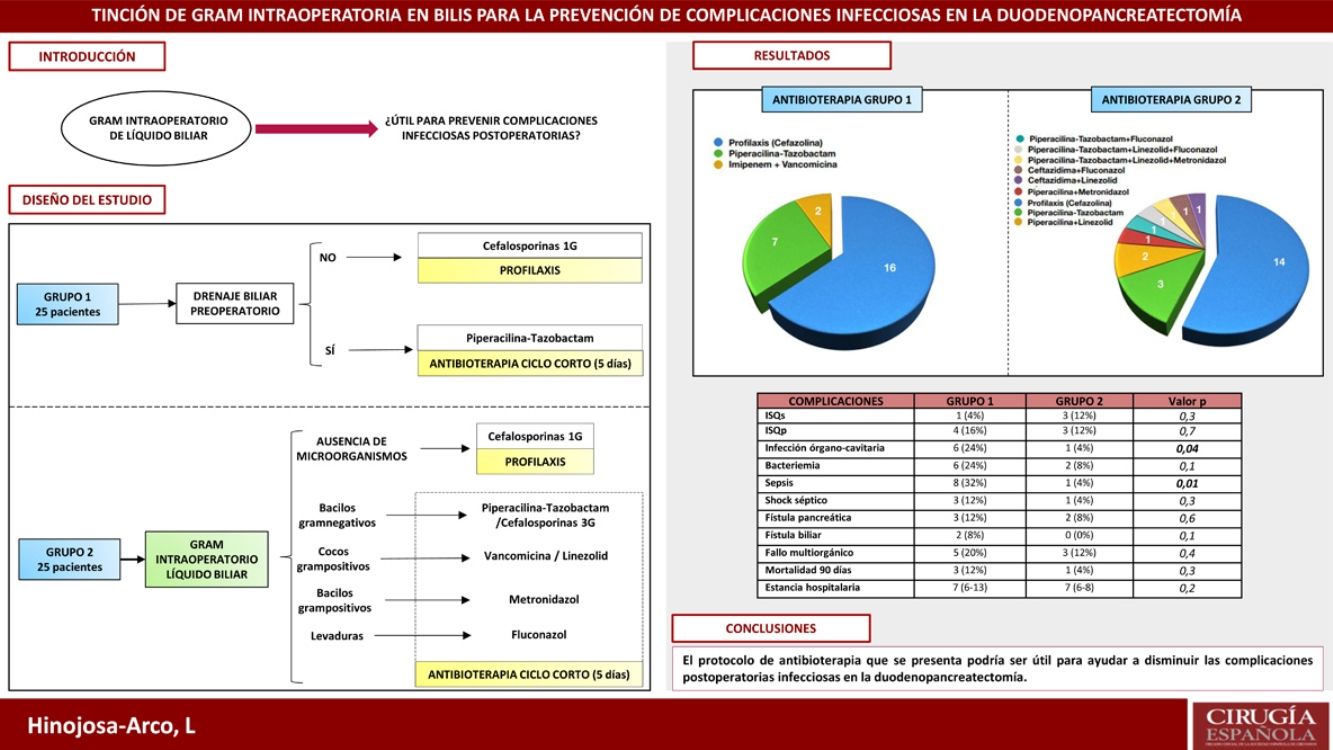
Infectious complications play a prominent role in pancreaticoduodenectomy. Their incidence increases in cases with preoperative biliary drainage (PBD), due to the higher risk of bacterobilia. The aim of this study is to evaluate an antibiotherapy protocol based on intraoperative gram staining of bile and its impact on postoperative infectious complications.
MethodsA retrospective study analysing the incidence of infectious complications between two groups of 25 consecutive patients undergoing pancreaticoduodenectomy. In group 1, cefazolin prophylaxis was administered to patients without PBD. In cases with PBD a five days antibiotherapy with piperacillin-tazobactam was administered. In group 2, intraoperative gram staining of bile was routinely performed. If no microorganisms were detected, antibiotherapy was limited to cefazolin prophylaxis. If bacterobilia was detected, targeted antibiotherapy was administered for five days.
ResultsThe incidence of sepsis and organ/space infection in group 2 was 4% compared to 32% and 24% in group 1 respectively (p < 0.05). No differences were observed in the remaining morbimortality variables. The most prevalent microorganisms in bile were Enterococcus spp. and Klebsiella spp. In postoperative samples, they only appeared in 4% of cases in group 2 (p < 0.05), in favour of S. epidermidis, although they were also prevalent in group 1 (28 and 24% respectively).
ConclusionIntraoperative gram staining of bile fluid could be a useful tool to conduct personalised antibiotic therapy in pancreaticoduodenectomy and contribute to the control of infectious complications.
Las complicaciones infecciosas presentan un papel destacado en la duodenopancreatectomía. Su incidencia aumenta en casos con drenaje biliar preoperatorio (DBP), por el mayor riesgo de bacterobilia. Se presenta un estudio con el objetivo de valorar un protocolo de antibioterapia guiado por una tinción de gram intraoperatoria de líquido biliar.
MétodosEstudio retrospectivo en el que se analiza la incidencia de complicaciones infecciosas entre dos grupos de 25 pacientes, consecutivos en el tiempo, intervenidos de duodenopancreatectomía. En el grupo 1 se administró profilaxis con cefazolina en pacientes sin DBP y antibioterapia durante cinco días con piperacilina-tazobactam en casos con DBP. En el grupo 2 se realizó tinción de gram intraoperatoria de bilis de forma sistemática. Si no se detectaban microorganismos, la antibioterapia se limitaba a profilaxis con cefazolina. Si se apreciaba bacterobilia, se administraba antibioterapia dirigida durante cinco días.
ResultadosLa incidencia de infección órgano-cavitaria fue del 24% en el grupo 1 y del 4% en el 2 (p = 0,04) y la incidencia de sepsis fue del 32% en el primer grupo y del 4% en el segundo (p = 0,01). No se apreciaron diferencias en el resto de variables de morbimortalidad. Los microorganismos más prevalentes en bilis fueron Enterococcus spp. y Klebsiella spp. En cultivos postoperatorios, aunque también fueron los más frecuentes en el grupo 1 (28 y 24%), solo aparecieron en el 4% de los casos del grupo 2 (p < 0,05).
ConclusiónLa tinción de gram intraoperatoria de bilis podría ser útil para dirigir la antibioterapia en la duodenopancreatectomía y contribuir a reducir las complicaciones infecciosas.
Duodenopancreatectomy (DP) is a surgical procedure for the treatment of periampullary tumours. Although postoperative mortality has decreased in recent decades, morbidity still has an incidence of 30%–60%1–3. Infectious complications play an important role1,4, and the presence of microorganisms in the bile at the time of surgery is a risk factor for their occurrence.3,5,6. This circumstance, known as bacterobilia, is favoured by preoperative biliary drainage (PBD), especially by endoscopic retrograde cholangiopancreatography (ERCP)7–9.
Perioperative antibiotic therapy plays a fundamental role in the prevention of this type of complications, including surgical site infection (SSI) and sepsis1,5,10. While prophylaxis has been the most recommended option, some authors propose the maintenance of antibiotic treatment during the first postoperative days, especially in patients at risk of bacterobilia1,10,11.
In accordance with this statement, our department used short-course antibiotic therapy from the time of surgery in patients with a history of PBD, administering only prophylaxis in the rest. However, the persistence of postoperative infectious complications prompted a change in clinical practice. In this context, routine intraoperative Gram staining of bile fluid was proposed. It was hypothesised that this tool would make it possible to detect the presence of bacterobilia at the time of surgery and, if necessary, to initiate targeted antibiotherapy to reduce postoperative infections.
Under this assumption, we proposed a study with the aim of comparing the incidence of infectious complications between patients treated according to the pre-protocol and the Gram stain-targeted protocol. As secondary objectives we proposed the determination of the performance of this microbiological test to detect bacterobilia and the analysis of the most frequent microorganisms in bile in our institution.
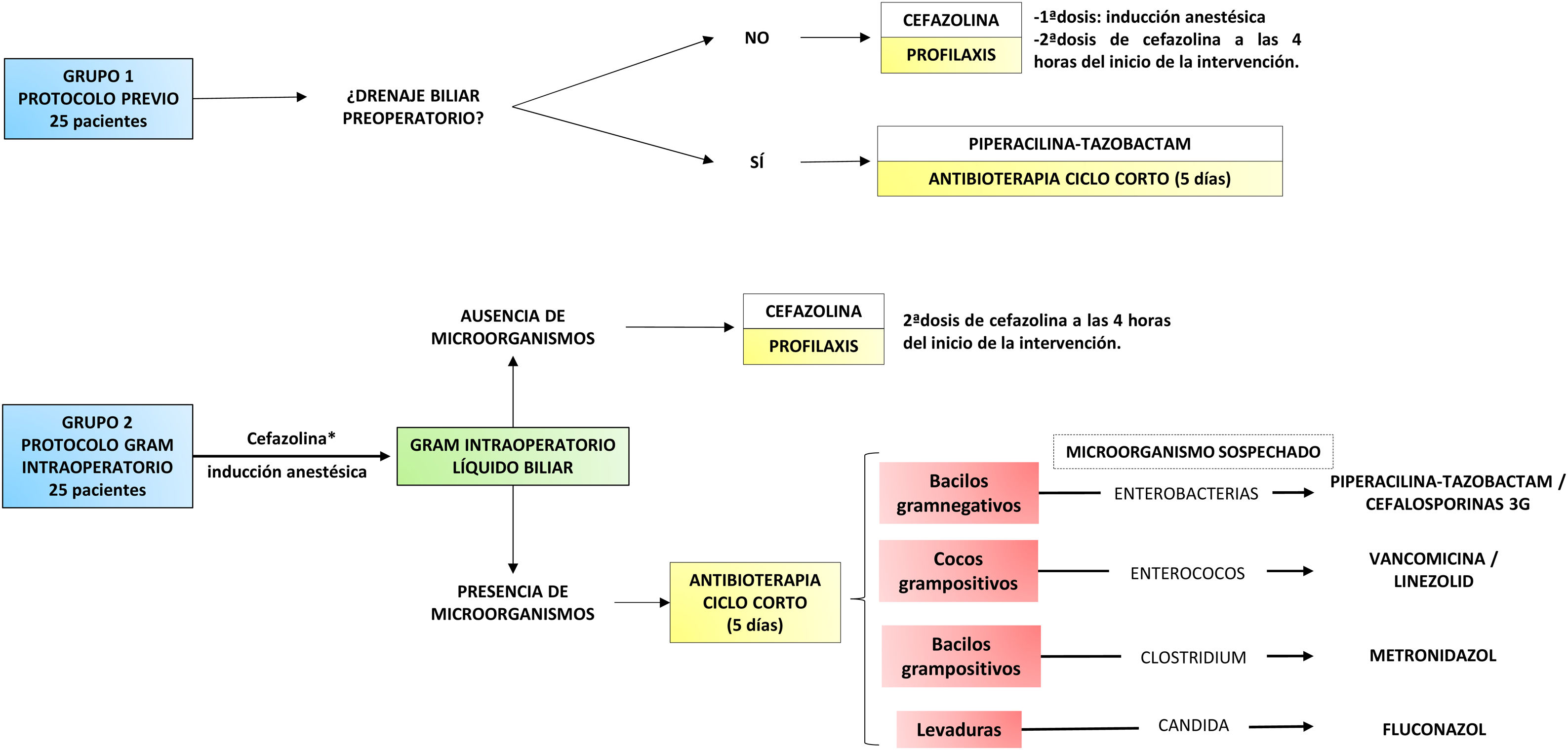
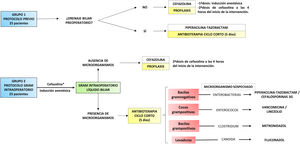
Material and methodsWe present an analytical, longitudinal, retrospective study carried out in a tertiary hospital centre. The study included patients who underwent cephalic duodenopancreatectomy (CDP) and total duodenopancreatectomy (TDP) between 2014 and 2018. Cases in which multivisceral resection (associated colectomy) was performed and those in which antibiotherapy protocol was not strictly adhered to or biliary culture data could not be retrieved were excluded. Thus, 50 patients were selected and divided into two groups (Fig. 1).
The first group included the last 25 cases treated with the previous protocol. In patients without PBD, cefazolin prophylaxis limited to the surgical procedure was administered. In cases with a history of PBD, short-course antibiotherapy with piperacillin-tazobactam was prescribed or, if recent microbiological cultures were available, an antibiotic adjusted to them. This treatment was maintained for 5 days and was discontinued as long as there was no intercurrent infectious process and it was accompanied by a decrease in reactive protein C compared to the first postoperative day. In all cases, a biliary fluid sample was taken for culture, and treatment was adapted to the results when available.
The second group included the first 25 cases treated with the protocol directed by Gram staining of bile fluid. First, a dose of antibiotic (cefazolin) was administered during anaesthetic induction12. Then, early in the procedure, a bile sample was taken by subcutaneous needle puncture of the common hepatic duct. This was sent to the microbiology laboratory, where, in addition to being processed for culture, it was immediately analysed by Gram staining. Once the result was obtained, in approximately one hour, the surgical team was informed. If no microorganisms were found, the administration of antibiotics was limited to prophylaxis. When the Gram stain identified a micro-organism, targeted antibiotic therapy with intent to treat was prescribed (Fig. 1). In the presence of several types of microorganisms, a combination of antibiotics could be administered. This treatment was maintained for 5 days and discontinued according to the same criteria as in the previous group. Similarly, it was adapted to the culture result if necessary.
All study participants are consecutive in time and were attended by the same medical team. In cases where CDP was performed, the anastomosis was pancreaticojejunal (hard pancreas and/or Wirsung >3 mm) or pancreatogastric (soft pancreas and/or Wirsung <3 mm). PBD was performed in the presence of cholangitis, renal failure or malnutrition associated with jaundice. It was also indicated in cases with elevated bilirubin (>15 mg/dl) with no possibility of early surgery and in patients with jaundice requiring neoadjuvant treatment. The preferred technique was ERCP or, if this was not possible, percutaneous transhepatic cholangiography (PTHC).
The following demographic variables were recorded for the study: age, sex, anaesthetic risk13, tumour characteristics and history of PBD (ERCP or PTCH). In relation to surgery, the following were recorded: type of intervention (CDP or TDP), pancreatic anastomosis, need for vascular resection and intraoperative transfusion of red blood cell concentrates.
Regarding the antibiotic therapy protocol, the following were recorded: perioperative antibiotics used (prophylaxis or short course treatment and, in this case, the duration of treatment) and the results of the Gram stain, bile fluid culture and postoperative microbiological sample cultures (blood culture, surgical wound and peritoneal fluid).
For the morbidity and mortality analysis, the following were recorded: development of pancreatic fistula14, biliary fistula15, delayed gastric emptying, haemorrhagic complications (intraluminal gastrointestinal bleeding and intra-abdominal bleeding), ischaemic complications (thrombosis of native blood vessels or prostheses) and multi-organ failure. The incidence of superficial SSI (sSSI), deep SSI (dSSI), organ-cavitary infection16, bacteraemia, sepsis and septic shock17 was also recorded. In addition, morbidity and mortality according to the Clavien-Dindo index18, hospital stay and postoperative mortality at 90 days were recorded.
SPSS-IBM® software was used for statistical analysis. Quantitative variables were expressed as median and interquartile range, and compared with the Mann Whitney-Wilcoxon U-test (age was expressed as mean and standard deviation and compared with the Student’s t-test, given that normal distribution was demonstrated by the Shapiro–Wilk test). Qualitative variables were expressed as absolute number and percentage and compared with the chi-square test. Differences were considered statistically significant when they presented a p < .05 value. To control for confounding variables, a backward stepwise logistic regression model was performed, assessing the odds ratio (OR) with 95% confidence intervals.
ResultsThe most relevant data from the descriptive analysis are shown in Table 1. It shows homogeneity of both groups, with no significant differences detected. Postoperative morbidity and mortality in each of the study groups is presented in Table 2. No differences were found in the occurrence of pancreatic fistula, biliary fistula, haemorrhagic complications, length of stay or mortality. With regard to infectious complications, the lower incidence of bacteraemia, septic shock, sepsis and organ-cavitary infection in the second group is noteworthy, with the latter two variables showing a significant relationship (p < .05).
Descriptive analysis. Characteristics of each of the study groups.
| Variables | Group 1 | Group 2 | p |
|---|---|---|---|
| Number of cases | 25 | 25 | 1,0 |
| Age | 65.8 (±11) | 64.5 (±7) | .6 |
| Sex | .7 | ||
| Woman | 10 (40%) | 11 (44%) | |
| Man | 15 (60%) | 14 (56%) | |
| Anaesthetic risk | .5 | ||
| ASA II | 12 (48%) | 11 (44%) | |
| ASA III | 12 (48%) | 14 (56%) | |
| ASA IV | 1 (4%) | 0% | |
| Tumour type | .5 | ||
| Adenocarcinoma of the pancreas | 16 (64%) | 15 (60%) | |
| Adenocarcinoma ampullae | 4 (16%) | 4 (16%) | |
| Cholangiocarcinoma | 0 (0%) | 2 (8%) | |
| Pancreatic cystic tumour | 3 (12%) | 1 (4%) | |
| Neuroendocrine tumour | 1 (4%) | 1 (4%) | |
| Metastatic renal carcinoma | 0 (0%) | 1 (4%) | |
| Chronic pancreatitis | 1 (4%) | 0 (0%) | |
| Leiomyoma ampullae | 0 (0%) | 1 (4%) | |
| Bacterobilia | 10 (40%) | 9 (36%) | .7 |
| Preoperative biliar drainage | 6 (24%) | 8 (32%) | .5 |
| ERPC | 6 (24%) | 7 (28%) | |
| PTCH | 0 (0%) | 1 (4%) | |
| Time between draining and surgery | 45 (29–49) | 42.5 (31–59) | .9 |
| Surgical intervention | 1.0 | ||
| CDP | 21 (84%) | 21 (84%) | |
| TDP | 4 (16%) | 4 (16%) | |
| Pancreatic anastomosis | .9 | ||
| No | 4 (16%) | 4 (16%) | |
| Pancreatico-jejunal | 11 (44%) | 12 (48%) | |
| Pancreatico-gastric | 10 (40%) | 9 (36%) | |
| Vascular resection | 5 (20%) | 2 (8%) | .2 |
| Intraoperative transfusion of red blood cell concentrates | 10 (40%) | 9 (36%) | .7 |
CDP: cephalic duodenopancreatectomy; ERCP: endoscopic retrograde cholangiopancreatography; PTC: percutaneous transhepatic cholangiography; TDP: total duodenopancreatectomy.
The quantitative variable “Time between drainage and surgery” is expressed as median and interquartile range (in brackets). It was calculated with the Mann Whitney-Wilcoxon U-test. The quantitative variable “Age” is expressed as mean and standard deviation (in brackets) and was calculated with Student’s t-test (given that normal distribution was verified by the Shapiro–Wilk test). Qualitative variables were expressed as absolute number and percentage (in brackets). They were calculated using the chi-square test.
Morbimortality of each study group.
| Complications | Group 1 | Group 2 | p |
|---|---|---|---|
| sSSI | 1 (4%) | 3 (12%) | .3 |
| dSSIp | 4 (16%) | 3 (12%) | .7 |
| Organ-cavity infection | 6 (24%) | 1 (4%) | .04 |
| Bacteraemia | 6 (24%) | 2 (8%) | .1 |
| Sepsis | 8 (32%) | 1 (4%) | .01 |
| Septic shock | 3 (12%) | 1 (4%) | .3 |
| Pancreatic fístula | 3 (12%) | 2 (8%) | .6 |
| Biliar fistula | 2 (8%) | 0 (0%) | .1 |
| Delayed gastric emptying | 1 (4%) | 1 (4%) | 1.0 |
| Haemorrhagic complications | 4 (16%) | 5 (20%) | .7 |
| Ischaemic complications | 1 (4%) | 0 (0%) | .3 |
| Multi-organ failure | 5 (20%) | 3 (12%) | .4 |
| Mortality 90 days | 3 (12%) | 1 (4%) | .3 |
| Hospital stay | 7 (6–13) | 7 (6–8) | .2 |
| Clavien-Dindo | .8 | ||
| 0 | 14 (56%) | 15 (60%) | |
| I | 0 (0%) | 0 (0%) | |
| II | 3 (12%) | 4 (16%) | |
| IIIa | 0 (0%) | 0 (0%) | |
| IIIb | 2 (8%) | 1 (4%) | |
| IV | 3 (12%) | 4 (16%) | |
| V | 3 (12%) | 1 (4%) |
dISQ: deep surgical site infection; sISQ: superficial surgical site infection.
The quantitative variable “Hospital stay” is expressed as median and interquartile range (in brackets). It was calculated with the Mann Whitney-Wilcoxon U-test. Qualitative variables were expressed as absolute number and percentage (in brackets). They were calculated with the chi-square test.
A history of PBD, the presence of bacterobilia and maintenance antibiotherapy were considered potential confounding variables. They were analysed with a backward stepwise logistic regression model where the dependent variable was organ-cavitary infection and the independent variable was the type of antibiotherapy protocol, controlling for potentially confounding variables. Intraoperative Gram-guided antibiotherapy acted as a protective factor against the development of organ-cavitary infection, with OR = .087 (.039–.508), independent of potential confounders. In addition, a history of PBD acted as a risk factor for the development of organ-cavitary infection, with OR = 6.9 (1.1–53.6). The same procedure was performed for the sepsis variable. Similarly, Gram stain-guided antibiotherapy proved to be a protective factor against the development of sepsis, with OR = .047 (.001–.372), independently of potential confounding variables. PBD also acted as a risk factor for the development of sepsis, with OR = 9.3 (1.5–82.3).
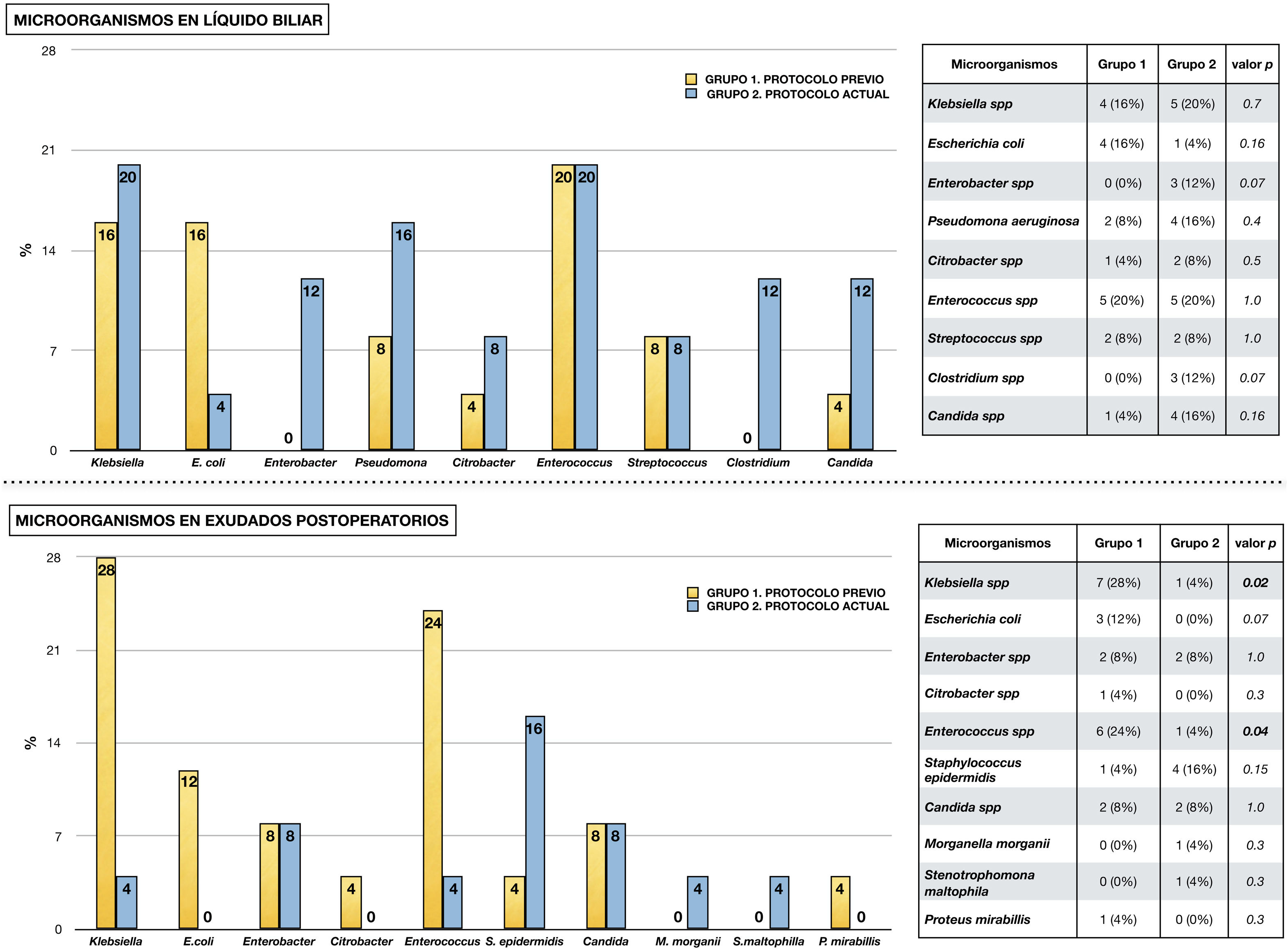
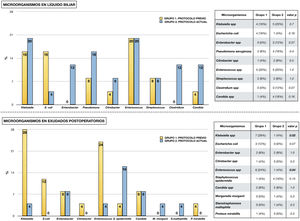
Fig. 2 shows the microorganisms found in the different microbiological samples in each group of the study. Diagram A shows the microorganisms detected in bile fluid, with the presence of Enterococcus spp. and Klebsiella spp. standing out in both groups. Diagram B shows the microorganisms found in postoperative cultures in relation to infectious complications. As in the bile, in group 1 the presence of Klebsiella spp., Enterococcus spp. and Escherichia coli stands out. In contrast, in group 2 a decrease in Enterococcus spp. and Klebsiella spp. was observed (p < .05), in favour of other microorganisms such as Staphylococcus epidermidis.
Micro-organisms isolated from microbiological samples.
The upper half shows the micro-organisms isolated from bile fluid in each study group. Table comparing each type of micro-organism by chi-square is attached.
The lower half shows the microorganisms isolated in microbiological samples taken postoperatively in the presence of infectious complications (surgical wound exudate, peritoneal fluid exudate and blood culture). A table comparing each type of microorganism using chi-square is attached.
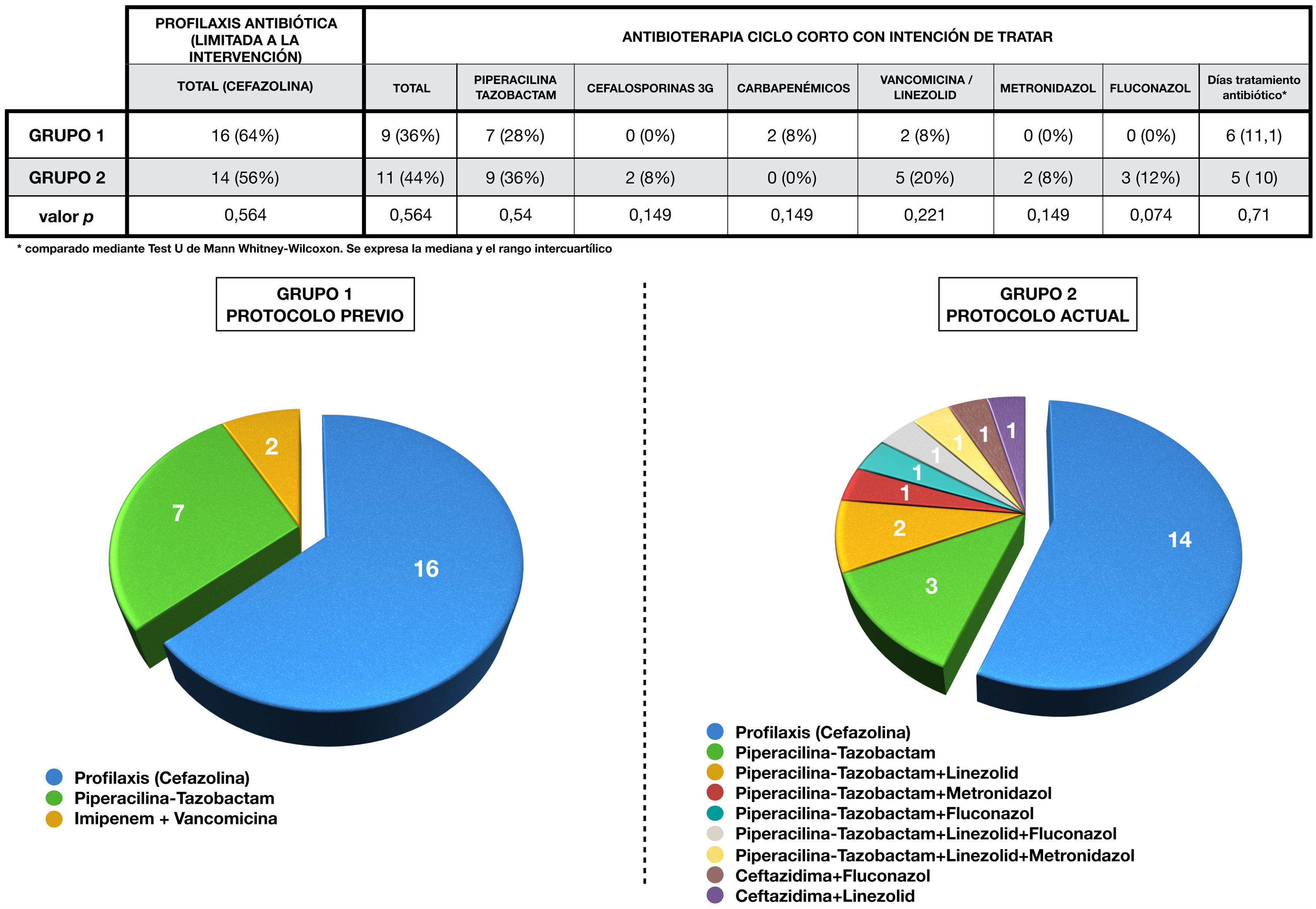
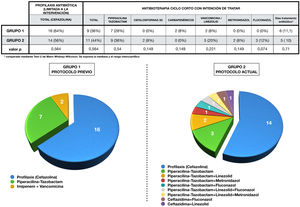
Antibiotic administration in each group is expressed in Fig. 3. Antibiotic therapy was limited to cefazolin prophylaxis in 64% (1, n = 16) of cases in group 1 and in 56% (1, n = 14) of group 2 (p = 0.6). On the other hand, short-course antibiotherapy was administered in 36% (1, n = 9) of patients in the first group and in 44% (1, n = 11) of the second (p = 0.6). The pie charts show the greater variability of drugs used in the 2 group, as well as their combinations.
Perioperative antibiotherapy administered in each group.
The upper part of the figure shows a table showing the antibiotic therapy in each group, compared using the chi-squared test.
The combinations of antibiotics used in each group during treatment, as well as the cases that only received prophylaxis, are represented by sector graphs.
Sensitivity analysis of microorganisms detected in bile revealed 60% of Enterococcus spp. resistant to piperacillin-tazobactam. The sensitivity of this group of microorganisms to vancomycin and linezolid was 100%. It should be noted that 50% of Enterococcus spp. corresponded to E. faecium. Resistance to piperacillin-tazobactam of Klebsiella spp. and Pseudomona aeuroginosa was also calculated, being 22.2% and 16.6%, and resistance of Klebsiella spp. to cefazolin, which was 77.7%. Also noteworthy was a rate of BLEE-producing gram-negative bacilli of 13%. On the other hand, the sensitivity of Clostridium spp. to piperacillin-tazobactam was 66.6%. When comparing a posteriori the antibiogram of the bile cultures with the perioperative antibiotherapy used (prophylactic and intention-to-treat), we observed that 23 of the 25 patients (92%) in group 2 had received treatment covering all microorganisms since the intervention, a circumstance that was only fulfilled in 17 of the 25 patients (68%) in the group 1 (p = .03).
The calculation of the internal and external validity of the intraoperative Gram stain of bile fluid is shown in Table 3. It shows the results of sensitivity, specificity, positive and negative predictive value with respect to its ability to detect bacterobilia, gram-negative bacilli, gram-positive cocci, gram-positive bacilli and yeasts.
Internal validity and external validity of the Gram stain for the detection of micro-organisms in bile fluid.
| Sensitivity | Specificity | PPV | PNV | |
|---|---|---|---|---|
| Bacterobilia | 88% | 94% | 88% | 94% |
| Gram-negative bacilli | 71% | 94% | 83% | 89% |
| Gram-positive cocci | 66% | 95% | 80% | 90% |
| Gram-positive bacilli | 100% | 100% | 100% | 100% |
| Yeasts | 33% | 95% | 50% | 91% |
PNV: predictive negative value; PPV: predictive positive value.
Infectious complications occupy a prominent place in DP morbidity and mortality, causing an increase in hospital stay and healthcare costs3,19,20. They may occur in more than one third of cases, and are more frequent in patients with PBD3,4,6,21. In our study, although there were no differences in length of stay or mortality, there was a lower incidence of serious infectious complications, such as sepsis and organ-cavitary infection in the group 2.
The presence of bacterobilia at the time of the intervention is a risk factor for developing infectious complications3. Bile, which is normally sterile, can be colonised in cases of biliary obstruction or after manipulation of the sphincter barrier8,9,22–25. The contamination that occurs is usually polymicrobial and differs between institutions26. Despite this variability, bacteria of the genus Enterococcus spp. stand out for their frequency and clinical repercussions, appearing in 20%–74% of cases, and other microorganisms such as Klebsiella spp., Escherichia coli, Enterobacter spp. and Candida spp.5,7,22,26–29. Consistent with these data, the most frequent microorganisms in bile in our series were Klebsiella spp., Enterococcus spp., Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae and Candida spp.
Another aspect of interest is the comparison between micro-organisms present in bile and those isolated in postoperative cultures. Although variable, correlation rates of up to 59% have been described5,10, In our study, the most frequent microorganisms in bile follow a similar pattern in both groups, with the presence of Klebsiella spp. and Enterococcus spp. standing out. In postoperative cultures, although these microorganisms continue to be the most frequent in the 1 group, they present a low incidence in the 2 group.
One of the most controversial issues is the selection and duration of perioperative antibiotherapy. Although the administration of prophylaxis is standard practice, several studies have assessed the effects of antibiotic therapy maintained since surgery1,10,11. A common aspect of their conclusions is that prolonging antibiotic treatment in patients with bacterobilia seems to lead to a decrease in infectious complications. Similarly, a distinction is usually made between patients with a low and high risk of biliary infection in order to use broad-spectrum antibiotics in the latter1,29. Gavazzi et al.28 even suggest adding antibiotics with action against Enterococcus spp. given their high prevalence in biliary cultures. Regarding the use of antifungals, they are only recommended prophylactically in immunocompromised patients in critical care units29–31. It should be clarified that our study proposes a short course of antibiotic therapy that may require the combination of several drugs according to the intraoperative Gram score only in cases of biliary contamination. However, this practice cannot be standardised until validated by other studies. In one way or another, it is advisable to know the most frequent micro-organisms in each institution in order to adapt perioperative antibiotherapy32.
Limitations of the study include the retrospective design and sample size, which means that the proposals and results of the study should be considered with caution until confirmed by prospective, randomised studies. Similarly, it is debatable whether the presence of gram-positive cocci in bile is sufficient reason to initiate treatment with linezolid or glycopeptides. In our case, we opted for this antibiotherapy due to the high incidence of E. faecium. However, we recognise that this indication is subject to a specific epidemiological context. A similar analysis requires the combination of metronidazole and piperacillin-tazobactam when gram-negative and gram-positive bacilli are identified. Since the sensitivity of Clostridium spp. to piperacillin-tazobactam was 66.6%, the need to combine these two drugs could be reviewed. Another aspect to consider for rational drug use is to avoid standardisation of piperacillin-tazobactam against gram-negative bacilli in favour of other drugs such as third generation cephalosporins.
One of its strengths is that antibiotherapy is only considered in cases of biliary contamination confirmed by Gram staining. This technique also provides early information on groups of microorganisms, which allows targeted treatment from the time of intervention. Although this tool has previously been proposed for the diagnosis of intraoperative infection22,33, most of the studies that suggest antibiotherapy do so based on the risk of bacterobilia, without confirming the existence of biliary contamination1,10,11.
In conclusion, although the results presented in this study have limited validity due to their characteristics, we consider that intraoperative bile Gram staining is a useful technique for the early identification of biliary contamination and for establishing individualised antibiotherapy in PD.
FinancingThis research received no funding from any public or commercial sector agents or not-for-profit entities.
Conflict of interestsThe authors have no conflict of interests to declare in this study development and final outcome.
Our thanks to Rita Pérez (IBIMA) for her advice in the statistical analysis.
Please cite this article as: Hinojosa Arco LC, Roldán de la Rua JF, Carranque Chaves GÁ, Mora Navas L, de Luna Díaz R, Suárez Muñoz MÁ. Tinción de Gram intraoperatoria en bilis para la prevención de complicaciones infecciosas en la duodenopancreatectomía. Cir Esp. 2022;100:472–480.