Intraductal papillary mucinous neoplasm (IPMN) of the pancreas can progress from low-grade dysplasia to high-grade dysplasia and invasive carcinoma.
MethodsIn this single-center retrospective series, we analyze the clinicopathological features and long-term follow up of patients who underwent pancreatic resection for IPMN, from January 2009 to December 2019.
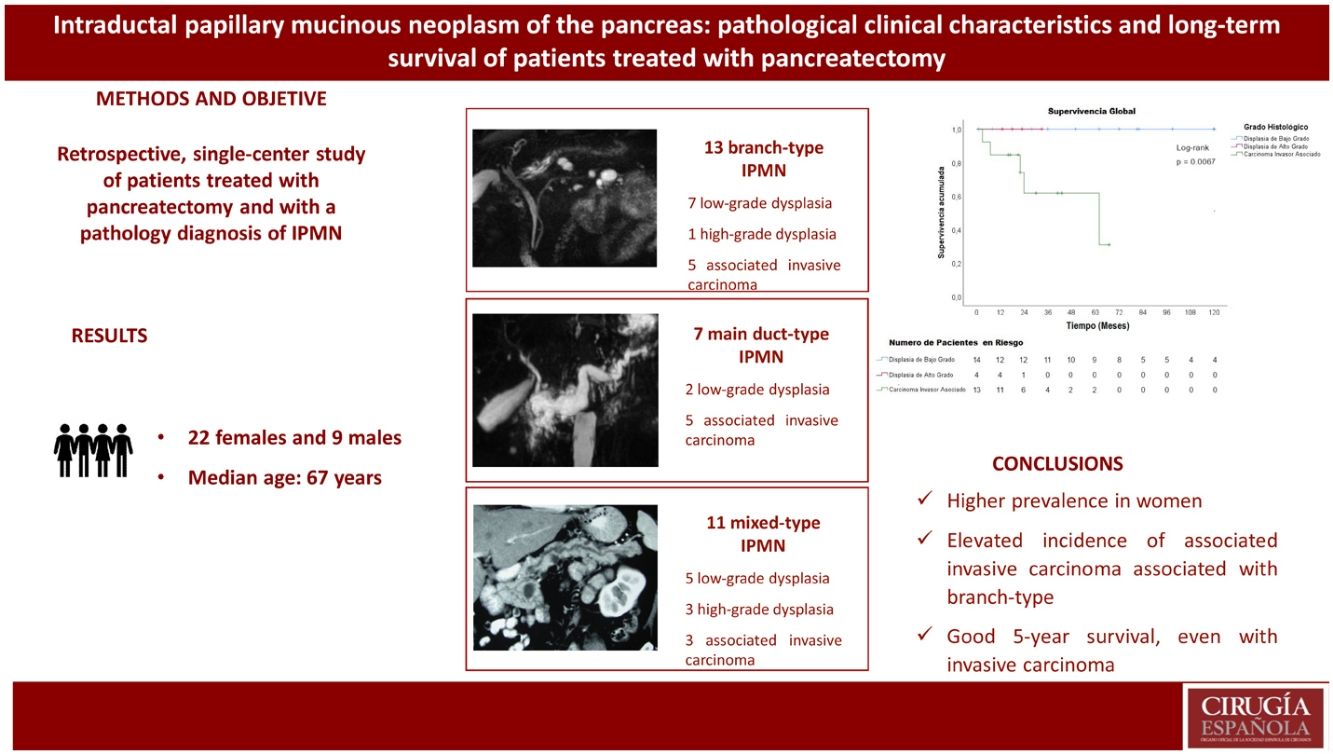
Results31 patients were diagnosed with IPMN: 9 males and 22 females. Mean age was 67 years. Twenty-seven patients (87%) were symptomatic. Seven patients had main duct IPMN, 11 branch-type IPMN and 13 mixed-type IPMN. High-risk stigmata were found in 20 patients (64.5%) and worrisome features in 10 patients (32.2%). Thirteen patients (41.9%) had an associated invasive carcinoma, 4 (12.9%) high-grade dysplasia and 14 (45.2%) low-grade dysplasia. The follow-up was from 2 to 12 years. Median survival for patients with IPMN and associated invasive carcinoma was 45.8 months, and disease-free survival was 40.8 months.
ConclusionsIPMN had a higher prevalence in females, mostly symptomatic and high incidence of associated invasive carcinoma with branch type. The 5-year survival was good even with associated invasive carcinoma.
La neoplasia mucinosa papilar intraductal (NMPI) del páncreas puede progresar de displasia de bajo grado a displasia de alto grado y carcinoma invasor asociado. El objetivo de este trabajo fue describir las características clínico-patológicas, y los resultados de seguimiento a largo plazo de pacientes con pancreatectomía por NMPI.
MétodosEn este estudio retrospectivo de un solo centro, se analizan los resultados de los pacientes sometidos a resección pancreática, con diagnóstico anatomo patológico de NMPI, desde enero del 2009 a diciembre del 2019.
Resultados31 pacientes tuvieron diagnóstico de NMPI. Nueve pacientes fueron varones y 22 mujeres. La edad media fue de 67 años. Veintisiete pacientes (87%) presentaron síntomas. Los estigmas de alto riesgo se encontraron en 20 pacientes (64.5%) y las características preocupantes (“worrisome features”) en 10 pacientes (32.2%). Siete pacientes tuvieron NMPI tipo conducto principal, 13 NMPI tipo rama y 11 NMPI tipo mixto. El carcinoma invasor asociado estuvo presente en 13 pacientes (41.9%), la displasia de alto grado en 4 pacientes (12.9%) y la displasia de bajo grado en 14 pacientes (45.2%). El tiempo de seguimiento fue de 2 a 12 años. La supervivencia media de los pacientes con NMPI y carcinoma invasor asociado fue de 45.8 meses y la supervivencia libre de enfermedad de estos pacientes fue de 40.8 meses.
ConclusionesEn nuestros pacientes operados, la NMPI tuvo mayor prevalencia en mujeres, fue predominantemente sintomática y tuvo una elevada incidencia de carcinoma invasor asociado a las de tipo rama. La supervivencia a 5 años fue buena aún con carcinoma invasor asociado.
Currently, efforts are being made to clarify the mechanisms of carcinogenesis in pancreatic cancer by studying 5 precursor lesions, which include: pancreatic intraepithelial neoplasia (PanIN), the most frequent1,2; intraductal papillary mucinous neoplasm; intraductal tubulopapillary neoplasm; intraductal papillary oncocytic neoplasm; and mucinous cystic neoplasm of the pancreas1,2,3.
Intraductal papillary mucinous neoplasm of the pancreas (IPMN) is a cystic lesion that originates from the epithelial cells lining the pancreatic ductal system and is characterized by cell proliferation forming papillary projections and mucin secretion4. These lesions may arise from the main pancreatic duct (main duct-type IPMN), its branches (branch-type IPMN), or both (mixed-type IPMN), and they can be solitary lesions or multifocal3. These neoplasms can progress from low-grade dysplasia to high-grade dysplasia and eventually invasive carcinoma5–7. This pathway of progression is presumed to account for 20%–30% of pancreatic cancers5. IPMN are an opportunity to identify a high-risk population that could benefit from preventive resection5.
Four decades after its first description, we now know that the risk of malignant progression is greater when the main duct is involved7. Approximately 23% of all resected IPMN have an associated invasive carcinoma8, specifically 43% for main duct-type IPMN, 45% for mixed-type IPMN, and 18.5% for branch-type IPMN, respectively9,10. Based on histology and mucin (MUC) expression, IPMN can be classified into 3 epithelial subtypes: gastric, intestinal, and pancreatobiliary; each are characterized by a different risk of malignant progression3,9,10. Gastric-type IPMN are usually lesions with low-grade dysplasia, while intestinal and pancreatobiliary lesions tend to be lesions with high-grade dysplasia and are frequently associated with invasive carcinoma5,7. Invasive carcinoma associated with IPMN can be ductal or colloid3,10, and the 5-year survival of these patients ranges from 34% to 62%, which is significantly higher compared to common ductal adenocarcinoma8. It is also known that ductal adenocarcinoma can appear in other parts of the pancreas in patients with IPMN, either synchronously or metachronously7. Finally, mutations in the KRAS and GNAS genes have been identified in IPMN, even before developing invasive carcinoma4.
The objective of this study was to describe the clinicopathological characteristics and long-term follow-up results of patients after pancreatectomy due to IPMN.
MethodsThis study is a retrospective, descriptive series of IPMN resected in a specialized Pancreas Surgery unit. From the database and with information collected prospectively, we identified patients with a pathological diagnosis of IPMN from January 2009 to December 2019.
We collected data for demographic, clinical, radiological, surgical, pathological and postoperative evolution variables. For the recurrence and survival analyses, electronic health records and follow-up radiological studies were reviewed retrospectively.
The diagnosis of IPMN was made according to World Health Organization (WHO) criteria: epithelial neoplasms of macroscopically visible mucus-producing cells (typically >5 mm) arising from the main pancreatic duct (main duct-type IPMN) and/or its branches (branch-type IPMN or mixed-type IPMN)3.
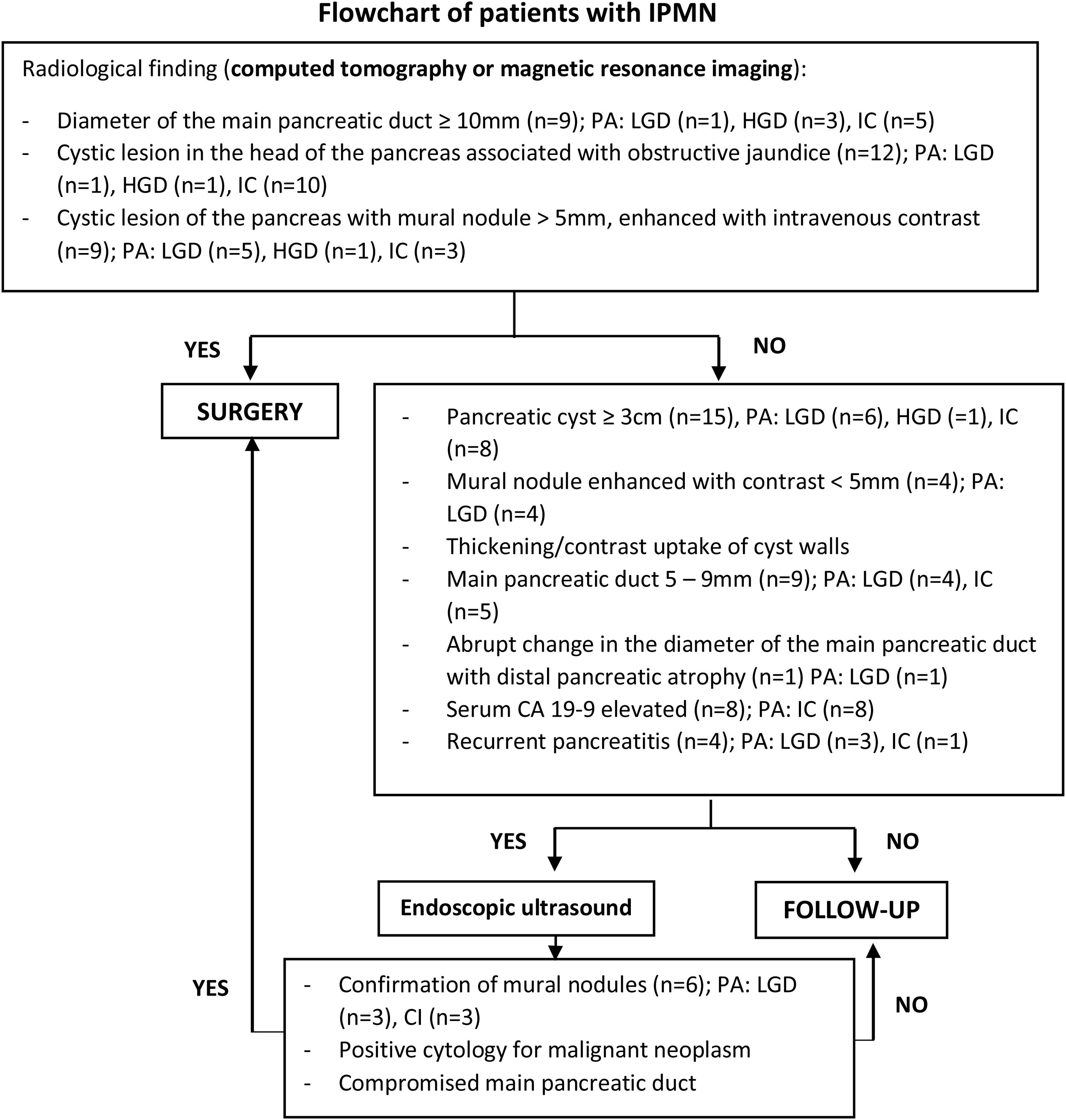
To identify patients with surgical indication and those who required further studies, we used the radiological criteria proposed by the International Association of Pancreatology (IAP)10, as follows:
- -
High-risk stigmata: obstructive jaundice in a patient with a cystic lesion in the head of the pancreas, mural nodule ≥5 mm that is enhanced with contrast, and main pancreatic duct ≥10mm10.
- -
Worrisome features: cyst ≥3 cm, contrast-enhanced mural nodule <5 mm, thickening/contrast enhancement of the cyst walls, main pancreatic duct 5–9 mm, abrupt change in caliber of the main pancreatic duct with distal pancreatic atrophy, lymphadenopathies, elevated serum CA 19-9 marker values, and rapid cyst growth >5 mm/2 years. Presence of pancreatitis in the clinical evolution10.
IPMN with low-grade dysplasia: characterized by the presence of mild-to-moderate atypia and may or may not have papillary projections and mitosis3,11,12
IPMN with high-grade dysplasia (carcinoma in situ): characterized by the presence of severe atypia, irregularly branching papillae, nuclear stratification with loss of polarity, pleomorphism, prominent nucleoli, and multiple mitoses3,11,12
IPMN with associated invasive carcinoma: the 2 possible types are colloid carcinoma and ductal adenocarcinoma3,10,12
IPMN with concomitant invasive carcinoma: Genetically distinct from IPMN in other parts of the gland, unlike associated invasive carcinoma. There should be a healthy transition zone of pancreatic parenchyma between IPMN and concomitant invasive carcinoma10,12
For the histopathological classification of the papilla type, the expression of membrane-bound mucins (MUC) was used3:
- -
Gastric-type IPMN: MUC1 (−), MUC2 (−), MUC5AC (+), MUC6 (+)3
- -
Intestinal-type IPMN: MUC1 (−), MUC2 (+), MUC5AC (+), MUC6 (−)3
- -
Pancreatobiliary-type IPMN: MUC1 (+), MUC2 (−), MUC5AC (+), MUC6 (+)3
Serum CA 19-9 values ≤37 U/mL were considered normal13. Patients with hyperbilirubinemia were excluded.
Preoperative indications are shown in detail in Fig. 1.
All pancreatic resections were performed with oncological criteria and standard lymphadenectomy.
Postoperative morbidity was defined as any local or general (systemic) postoperative complication within the first 90 days of the postoperative period. Pancreatic fistula was defined as clinically relevant grade B or C in accordance with the International Study Group on Pancreatic Fistula classification14. Post-pancreatectomy hemorrhage was determined as any episode of grade B or C postoperative hemorrhage, as defined by the International Study Group of Pancreatic Surgery classification15. Surgical site infection was determined according to the Centers for Disease Control and Prevention (CDC) criteria16. Intra-abdominal abscess was established with the presence of clinical signs of sepsis (tachycardia, leukocytosis, fever), a tomographic finding of a collection, and a positive culture of the collection. The modified Clavien-Dindo classification was used to grade the complications17. Severe complications were defined as Clavien-Dindo grade III or above. Postoperative mortality was defined as that which occurred within 90 days after surgery or during hospitalization.
Statistical analysisStatistical analysis was performed using IBM SPSS Statistics version 25 for Windows. Discrete variables are expressed as frequencies and percentages, continuous variables are expressed as medians and interquartile range (IQR). Categorical variables were compared with the chi-squared test. The univariate analysis was performed to assess the factors associated with high-grade dysplasia and invasive carcinoma. The variables that had significant statistical correlation in the univariate analysis were included in the multivariate analysis. A P value <.05 was considered statistically significant. The Kaplan-Meier method was used to calculate survival.
The study was conducted in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines18. It also complies with current regulations on bioethical research and was authorized by the Ethics Committee of our institution.
ResultsDuring the study period, 399 pancreatic resections were performed (320 pancreaticoduodenectomies and 79 distal pancreatectomies), 31 of which (7.7%) had a pathological diagnosis of IPMN. Nine patients were male and 22 were female. The mean age of the men was 64.3 years and of the women 69 years. Twenty-seven patients (87%) presented symptoms, the most frequent being abdominal pain in 20 patients (64.5%), followed by weight loss in 10 patients (32.3%). Jaundice was observed in 12 patients (38.7%), 11 of whom had associated invasive carcinoma and one low-grade dysplasia. Pancreatitis was present in 4 patients (12.9%), one of which had associated invasive carcinoma and 3 low-grade dysplasia (Table 1).
Clinical-pathological characteristics of resected IPMN.
| Characteristics | Total (n = 31) | Low-grade dysplasia (n = 14) | High-grade dysplasia (n = 4) | Associated invasive carcinoma (n = 13) |
|---|---|---|---|---|
| Age (yrs), median (IQR) | 67 (11.5) | 68.5 (9.5) | 69 (13.8) | 66 (10) |
| Males/Females | 9/22 | 4/10 | 0/4 | 5/8 |
| Symptomatic | 27 | 12 | 3 | 12 |
| Incidental finding | 4 | 2 | 1 | 1 |
| Associated neoplasm | 4 | 2 | 0 | 2 |
| CA19-9 (U/mL), median (IQR) | 12 (35.99) | 10.8 (11.2) | 4.69 (3.48) | 768 (752) |
| Tomography | 31 | 14 | 4 | 13 |
| Magnetic resonance | 28 | 14 | 4 | 10 |
| Endoscopic ultrasound | 12 | 11 | 0 | 1 |
| Fine-needle aspiration | 5 | 5 | 0 | 0 |
| CEA (ng/mL) intracystic, median (IQR) | 416 (391) | 416 (391) | ||
| High-risk stigmata | 20 | 3 | 4 | 13 |
| Worrisome features | 10 | 10 | 0 | 0 |
| IPMN location | ||||
| Head/uncinate process | 24 | 9 | 3 | 12 |
| Body/tail | 3 | 2 | 1 | 0 |
| Multifocal | 4 | 3 | 0 | 1 |
| Presence of mural nodules | 9 | 5 | 1 | 3 |
| Surgical procedure | ||||
| Pancreatoduodenectomy | 23 | 9 | 3 | 11 |
| Distal pancreatectomy | 7 | 5 | 1 | 1 |
| Total pancreatectomy | 1 | 0 | 0 | 1 |
| Postoperative complications (Clavien-Dindo ≥ III) | 9 | 4 | 1 | 4 |
| Re-operation | 2 | 2 | 0 | 0 |
| Deaths | 1 | 1 | 0 | 0 |
| Hospital stay (days), median (IQR) | 16.7 (8) | 16.8 (7.5) | 13.8 (8.75) | 17.5 (7.0) |
| Type of IPMN | ||||
| Main duct | 7 | 2 | 0 | 5 |
| Branch | 13 | 7 | 1 | 5 |
| Mixed | 11 | 5 | 3 | 3 |
| Resected lymph nodes | 11 | 10 | 17 | 13 |
| Resection margin involvement | 2 | 0 | 1 | 1 |
IPMN: intraductal papillary mucinous neoplasm; IQR: interquartile range; CA 19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen.
All patients underwent tomography as part of the preoperative studies, and 28 patients underwent magnetic resonance imaging (MRI) (Fig. 1). Endoscopic ultrasound was performed in 12 patients (38.7%) and fine-needle aspiration in 5 patients (16.1%). The median CEA value of the intracystic fluid obtained by fine-needle aspiration was 416 ng/mL (range 121–731 ng/mL). Only one patient had a value less than 192 ng/mL (Table 1).
High-risk stigmata were found in 20 patients (64.5%), and worrisome features were observed in 10 patients (32.2%). The most frequent location of the IPMN was in the head of the pancreas in 24 patients (77.4%). In total, 23 pancreatectomies, 7 distal pancreatectomies with splenectomy (3 laparoscopic and 4 open) and one total pancreatectomy were performed.
Immunohistochemistry with MUC was performed in 4 patients (13%), 2 of whom had intestinal-type IPMN (one associated with invasive colloid-type carcinoma and the other with high-grade dysplasia) and 2 pancreatobiliary-type IPMN (one associated with invasive ductal carcinoma and the other with high-grade dysplasia).
Associated invasive carcinoma was present in 13 patients (41.9%), high-grade dysplasia in 4 patients (12.9%), and low-grade dysplasia in 14 patients (45.2%). Among the 7 patients with main duct-type IPMN, 5 patients had associated invasive carcinoma (71.4%). Among the 11 patients with mixed-type IPMN, 3 had associated invasive carcinoma (27%); meanwhile, among the 13 patients with branch-type IPMN, 5 patients had associated invasive carcinoma (38.5%) (Table 2).
IPMN resected with associated invasive carcinoma.
| Total | Tubular adenocarcinoma(Ductal) | Colloid carcinoma | |
|---|---|---|---|
| Patients | 13 | 10 | 3 |
| Morphological type | |||
| IPMN main duct/mixed type | 8 | 5 | 3 |
| Branch-type IPMN | 5 | 5 | 0 |
| T (AJCC) | |||
| T1a | |||
| T1b | |||
| T1c | |||
| T2 | 6 | 6 | |
| T3 | 7 | 4 | 3 |
| T4 | |||
| N (AJCC) | |||
| N0 | 10 | 7 | 3 |
| N1 | 1 | 1 | |
| N2 | 2 | 2 | |
| Final stage (AJCC) | |||
| IA | |||
| IB | 4 | 4 | |
| IIA | 6 | 3 | 3 |
| IIB | 1 | 1 | |
| III | 2 | 2 | |
| IV | |||
IPMN: intraductal papillary mucinous neoplasm; AJCC: American Joint Committee on Cancer.
Synchronous concomitant carcinoma was present in only one patient (3.2%) with branch-type IPMN, and it was a ductal adenocarcinoma.
Median follow-up time was 33 months, ranging from one month (one patient died from postoperative complications) to 144 months (12 years). Mean survival of patients with IPMN and associated invasive carcinoma was 45.8 months, and the mean disease-free survival of these patients was 40.8 months (Figs. 2 and 3). Survival of patients with invasive tubular carcinoma was 45 months, while the survival of patients with colloid carcinoma was 52 months (P = .84).
DiscussionA multicenter study carried out in more than 100 hospitals in the United States reported that 10% of pancreatic resections were performed for IPMN19. In our department, 7.7% of the pancreatic resections carried out during the study period were for IPMN.
In the present series, the average age at the time of surgery was 67 years, a value similar to other contemporary series of resected IPMN, which report a mean age of 69 years at the time of surgery20, ranging from 40 to 75 years21. Likewise, a higher prevalence was found in women; this is unlike most series, which reported a slightly higher prevalence in men21,22.
Most patients in our study presented symptoms, and pain was the most frequent. This finding is also contrary to other series, in which the incidental finding is more frequent21,23. When patients present symptoms, abdominal pain is the most frequent (35%), followed by weight loss (29%), jaundice (11%)20, onset of diabetes or worsened symptoms, steatorrhea21 and acute pancreatitis (13%–32%)24. It is believed that the abundant mucin produced by IPMN could cause ductal obstruction, resulting in acute pancreatitis25 that is mostly mild. However, necrotizing pancreatitis has also been described in up to 4%26. Pancreatitis is currently considered a worrisome feature and is an indication for surgery to avoid eventual severe pancreatitis10,25. In this series, 4 patients (13%) had acute pancreatitis, all mild.
The diagnosis of IPMN is based on radiological studies (computed tomography or magnetic resonance imaging using gadolinium and pancreatocholangiography) and endoscopic ultrasound. These studies have different objectives: first, to differentiate IPMN from other cystic lesions of the pancreas; second, to determine the type of IPMN; and third, to identify the characteristics associated with malignancy21. Magnetic resonance imaging is more sensitive than tomography in identifying communication between the cyst and the pancreatic ductal system, which is characteristic of IPMN; it can also identify multiple cysts, nodules, thickened walls, and the size of the main pancreatic duct21. More recently, diffusion restriction on MRI has been proposed as a radiological feature of IPMN associated with invasive carcinoma27.
Endoscopic echocardiography is considered a “second-level” diagnostic study, after magnetic resonance imaging and tomography10,21. This procedure helps differentiate IPMN from macrocystic serous cystic neoplasm and pancreatic pseudocyst through the analysis of cyst fluid (CEA, amylase/lipase, glucose, and cytology)21. Its use is recommended in patients with worrying characteristics and those without a clear diagnosis10 (Fig. 1). Fluid CEA values greater than 192 ng/mL differentiate mucinous from non-mucinous neoplasms10,21 with a sensitivity of 38%-78% and a specificity of 63%-99%, but they do not help differentiate between benign or malignant neoplasms21. Cytology of cystic fluid can assist the diagnosis, although with low sensitivity due to low cellularity10. The molecular analysis of the fluid is still under development, while KRAS and GNAS gene mutations help reach the diagnosis of mucinous cysts and recognize the indolent behavior of cysts that could be observed, respectively10. In the present series, endoscopic ultrasound was performed in 12 patients due to uncertain diagnosis, and fine-needle aspiration was carried out in 5 cases because the communication of the cystic lesion with the pancreatic ductal system could not be clearly demonstrated by MRI, obtaining a CEA value >192 ng/mL in 75% of cases. Endoscopic ultrasound also makes it possible to identify true mural nodules and differentiate them from mucus plugs, especially when contrast-enhanced harmonic imaging is used, with a sensitivity of 60%–100% and a specificity of 75%–93%28. Among the patients in this study, mural nodules were identified by endoscopic ultrasound in 3 cases with low-grade dysplasia and in 3 cases with associated invasive carcinoma. These results are probably due to the fact that we do not have contrast-enhanced harmonic imaging in our setting.
Elevated serum CA 19-9 values are also found in patients with IPMN and associated invasive carcinoma in up to 63%28,29, with a sensitivity of 34.2%–52% and a specificity of 89%–92%30,31. In this series, 61% of patients with IPMN and associated invasive carcinoma had high levels of this marker.
Considering that surgery is indicated in IPMN with a potential risk of cancer, surgical treatment should consist of oncological resection with standard lymphadenectomy32. These pancreatic resections have higher morbidity than other gastrointestinal surgeries as well as long-term complications, such as endocrine and exocrine pancreatic insufficiency, or the development of fatty liver disease33. For these reasons, surgery is not recommended in all patients with IPMN. Ideally, resection should be done when the IPMN has high-grade dysplasia21,29,33,24, although it is difficult to make this diagnosis preoperatively33. In the present series, 12.9% of patients had high-grade dysplasia at the time of resection, and 41.9% had IPMN with associated invasive carcinoma; 45.2% had low-grade dysplasia, and 7 patients were treated surgically for Wirsung dilation, 5 for presence of mural nodules, and the other 2 cases for the lack of a clear diagnosis.
There is no doubt that all main duct or mixed-type IPMN should be managed surgically9,10 due to the high percentage of associated invasive carcinoma. What is difficult is to determine when to resect a branch-type IPMN, which has a risk of associated invasive carcinoma from 16.5%–18.5%9,10,21. In addition, we must bear in mind that the percentage of malignancy of branch-type IPMN is overestimated because these values are based on series of resected IPMN, while currently most of these patients are managed with observation and follow-up protocols. As a consequence, the incidence of invasive carcinoma associated with branch-type IPMN is probably less than 5%21. A 20-year follow-up study of patients with branch-type IPMN found that the 5-year incidence of associated invasive carcinoma was 3.3%, reaching 12% by 15 years35. In our study, we found an incidence of invasive carcinoma associated with branch-type IPMN of 38.5%, which is high compared to contemporary series. This is probably due to the small number of patients resected or to a different behavior of this pathology in our study population. Although branch-type IPMN resection certainly deserves consideration, we must keep in mind that these lesions occur in elderly patients, and the annual rate of progression to high-grade dysplasia or invasive carcinoma is relatively low (1.4%–6.9%)10. This allows us to observe and follow up on cases that do not have predictors of invasive carcinoma or high-grade dysplasia.
Jaundice, contrast-enhanced mural nodules or solid component, and dilation of the main pancreatic duct ≥10 mm all have a predictive value for malignancy of 56%–89%32. A current study of the high-risk stigmata and worrisome features proposed by the IAP found that the presence of jaundice, cyst size larger than 3 cm, solid component or mural nodules, pain as a symptom, and weight loss were significantly associated with IPMN with high-grade dysplasia and invasive carcinoma36. In the present series, the univariate analysis of the statistically significant factors associated with high-grade dysplasia and invasive carcinoma were jaundice and a CA19-9 value >37U/mL; however, in the multivariate analysis, only jaundice was statistically significant (Table 3).
Univariate and multivariate analysis of factors associated with high-grade dysplasia and invasive carcinoma.
| IPMN with low-grade dysplasia | IPMN with high-grade dysplasia/invasive carcinoma | P value | Univariate analysis OR (95%CI) | P value | Multivariate analysis OR (95%CI) | |
|---|---|---|---|---|---|---|
| Jaundice | 1 | 11 | 0.001* | 23.8(2.48−229) | 0.009* | 26(2.22−305) |
| Mural nodules | 5 | 4 | 0.109 | |||
| Wirsung > 10 mm | 1 | 8 | 0.093 | |||
| Wirsung 5–9 mm | 4 | 5 | 0.317 | |||
| Tumor size greater than 30 mm | 6 | 9 | 0.093 | |||
| Increase in CA19−9 > 37 U/mL | 0 | 8 | 0.003* | 25.9(1.33−504) | 0.995 | |
| Pancreatitis | 3 | 1 | 0.199 |
IPMN: intraductal papillary mucinous neoplasm, OR: odds ratio, CI: confidence interval, *:P < .05, CA 19-9: carbohydrate antigen.19-9.
Carcinomas associated with IPMN, as well as concomitant adenocarcinoma, have a better prognosis compared to common pancreatic adenocarcinoma4. Mean survival of IPMN associated with invasive carcinoma is 76.6 months37, and mean disease-free survival is 60.3 months38. Some studies have shown 5-year survival rates of 55% for tubular carcinoma and 87% for colloid carcinomas, with a mean of 23-26 months for tubular carcinoma and 91–127 months for colloid carcinoma4,39. In our study, mean survival of patients with IPMN and associated invasive carcinoma was 45.8 months, and the mean disease-free survival of these patients was 40.8 months (Figs. 2 and 3). Survival was longer in patients with associated colloid carcinoma compared with tubular carcinoma (52 months versus 48 months).
Recurrence after resection of an IPMN with low- and high-grade dysplasia ranges from 0% to 17% and can reach up to 43.3% after resection of an associated invasive carcinoma32,34. Therefore, lifelong surveillance of the pancreatic remnant is recommended.
The limitations of this study are: its retrospective design, the inclusion of only patients with resected IPMN, the limitation of resources such as contrast-enhanced harmonic endoscopic ultrasound, as well as the relatively small number of patients. We must also take into account that, although the study was conducted using recent concepts, this pathology has seen changes in its definition, classification and indications for resection and follow-up during the study period.
Until more reliable tools are developed to determine when to resect an IPMN, especially the branch-type, the treatment of this pathology should be based on multidisciplinary discussions, individualizing each patient and reviewing existing guidelines.
In conclusion, IPMN of the pancreas is a heterogeneous and potentially malignant disease, with a higher prevalence in women. In our surgical patients, the presentation was predominantly symptomatic, and there was a high incidence of invasive carcinoma associated with branch-type lesions.
Despite current treatment guidelines, making an exact preoperative diagnosis is difficult, and the risks of pancreatic surgery and the malignant potential of this disease often have to be assessed.
The 5-year survival outcome of patients with resected IPMN is good, even with associated invasive carcinoma.
FundingThe study has received no specific funding from public, commercial, or non-profit sectors.
Conflict of interestsNone.