Surgery and chemotherapy have increased the survival of pancreatic cancer. The decrease in postoperative morbidity and mortality and increase in life expectancy, has expanded the indications por cephalic pancreaticoduodenectomy (PDC), although it remains controversial in the geriatric population.
MethodsRetrospective study on a prospective database of patients with ductal adenocarcinoma of pancreas who underwent PDC between 2007–2018. The main objective was to analyse the morbidity-mortality and survival associated with PDC in patients ≥75 years (elderly).
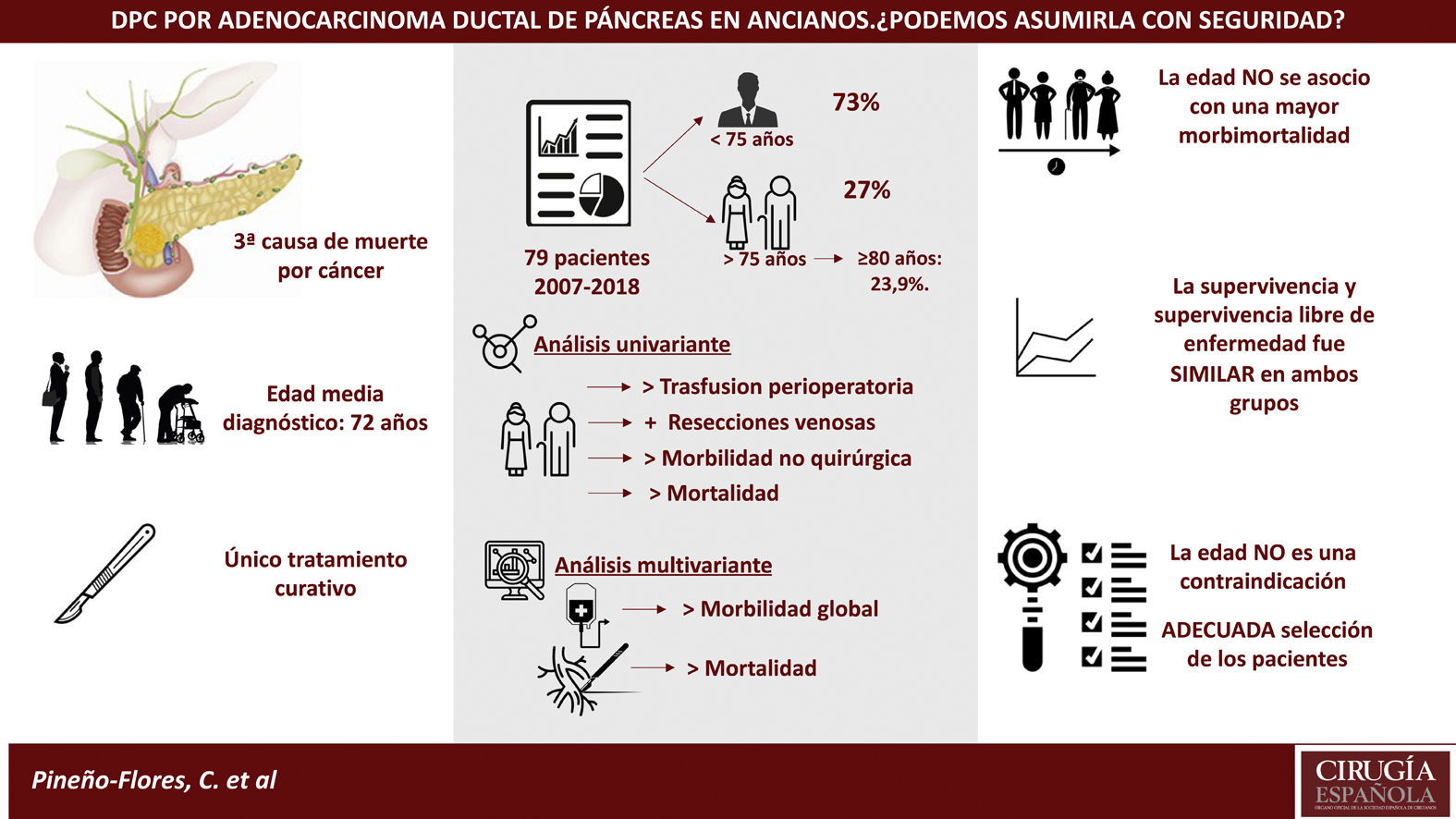
Results79 patients were included, 21 of them older than 75 years (27%); within this group, 23’9% were over 80 years old. The ASA of both groups was similar. Patients ≥75 years required more transfusions. No differences in operating time were observed, although more vascular resection were performed in the elderly (26 vs. 8.7%; P = .037).
Morbidity was higher in the elderly (61.9% vs. 46.6%), although without differences. Patients aged ≥75 years had more non-surgical complications (33.3%, P = .050), being pneumonia the most frequent. Postoperative mortality was higher in the ≥75 years (9 vs. 0%; P = .017). The overall survival and disease-free survival did not show significant differences in both groups.
ConclusionsElderly patients had higher postoperative mortality and more non-surgical complications. Survival did not show differences, so with an adequate selection of patients, age should not be considered itself as a contraindication for PDC.
La cirugía y quimioterapia han aumentado la supervivencia de los pacientes con neoplasias pancreáticas. La disminución de la morbi-mortalidad postoperatoria y el aumento de la esperanza de vida han ampliado las indicaciones de la duodenopancreatectomía cefálica (DPC), aunque sigue siendo controvertida en la población geriátrica.
MétodosEstudio observacional retrospectivo sobre una base de datos prospectiva, de pacientes con adenocarcinoma ductal de páncreas sometidos a una DPC entre 2007–2018. El objetivo principal fue analizar la morbi-mortalidad y supervivencia asociada a la DPC en pacientes ≥75 años (ancianos).
ResultadosSe incluyeron 79 pacientes, 21 de ellos mayores de 75 años (27%); dentro de este grupo el 23,9% tenían más de 80 años. El ASA de ambos grupos fue similar. Los pacientes ≥75años requirieron más transfusiones. No se observaron diferencias en el tiempo operatorio, aunque en los ancianos se realizaron más resecciones vasculares (26 vs. 8,7%; p = 0,037).
La morbilidad fue mayor en los ancianos (61,9% vs. 46,6%), aunque sin diferencias. Los ≥75 años presentaron más complicaciones no quirúrgicas (33,3%, p = 0,050) siendo la neumonía la más frecuente. La mortalidad postoperatoria fue superior en los ≥75 años (9 vs.0%; p = 0,017), constituyendo la resección venosa un factor de riesgo (p = 0,01). La supervivencia global y supervivencia libre de enfermedad no mostró diferencias significativas en ambos grupos.
ConclusionesLos pacientes ancianos presentaron una mayor mortalidad postoperatoria y más complicaciones no quirúrgicas. La supervivencia no mostró diferencias, por lo que con una adecuada selección de pacientes, la edad no debe constituirse por sí misma como una contraindicación para la DPC.
The aging of the population of Spain is a known fact, and it is estimated that 20% of the population will be >65 years old by 20501. The incidence of cancer increases with age, reaching 23.5% in patients >75 years2. It is estimated that, by 2030, 75% of all neoplasms and 85% of cancer deaths will occur in patients over 65 years of age3,4. Likewise, the incidence of pancreatic cancer increases with age, with an average age at the time of diagnosis of 72 years; specifically, 25.4% are aged 65-74, 28.6% 75–84, and 13.3% >85 years of age5,6.
Pancreatic cancer is the third leading cause of cancer death behind lung and colorectal cancer. In Spain, it is estimated that 4276 men and 3893 women were diagnosed with pancreatic cancer in 2019. Ductal adenocarcinoma represents 85% of pancreatic cancers, with an overall 5-year survival rate of 6%7. Surgery, together with neoadjuvant chemotherapy, have increased the survival of patients with resectable disease, compared with unresectable types (12.6 vs 3.6 months). However, the resectability rate only reaches 20%–25%8.
Pancreaticoduodenectomy (PD) is the technique of choice in tumors of the head of the pancreas. The evolution of the surgical technique and postoperative care has reduced mortality from 25% in 1960 to less than 5% in high-volume hospitals. However, postoperative morbidity ranges between 40% and 70%5,6,9. The decrease in complications, together with the increase in life expectancy, have led to the expansion of the indications for PD, although its indication in the elderly is controversial due to the poor prognosis of the disease as well as the greater frailty and less functional reserve of this patient population.10.
Publications about pancreatic surgery in the elderly are heterogeneous. This affects everything from the definition of ‘elderly’ to the indication for surgery and the type of pancreatic resection, which makes it difficult to compare results4,10–15.
The main objective of this study was to analyze and compare the morbidity, mortality and survival rates of PD in patients over 75 years of age with pancreatic ductal adenocarcinoma compared to younger patients.
MethodsStudy design. We conducted a retrospective observational study with data from a prospective database of patients who had undergone PD for pancreatic ductal adenocarcinoma from 2007–2018. All patients were evaluated in a multidisciplinary pancreatic pathology committee and were divided into 2 age groups according to the World Health Organization classification: <75 years and ≥75 years11.
Evaluation of resectability. Tumor resectability was established by triphasic computed tomography, enhanced with intravenous contrast. Resectable, borderline, and unresectable tumors were identified in accordance with the resectability criteria of the NCCN-2017 guidelines16,17. Patients with a different histological type or a surgical technique other than PD were excluded.
Surgical technique. Resection included: antrectomy and lymph node dissection of the hepatoduodenal ligament, hepatic artery, and right lateral side of the superior mesenteric artery. Reconstruction was performed using a double Roux-en-Y loop, with end-to-side pancreaticojejunal anastomosis in 2 planes and end-to-side hepaticojejunostomy. All vascular resections were venous. Three types of vascular reconstructions were performed according to the degree of vascular infiltration: (1) lateral suture of the SMV/P if the invasion was <50% of the venous circumference; (2) segmental resection with autologous end-to-end anastomosis if the invasion was >50%; and (3) substitution with a polytetrafluoroethylene stent in one case with a 3 cm-long venous infiltration. Portal Doppler ultrasound was performed 24 h later in all patients with vascular resection. Two drains were left proximal to the pancreatic anastomosis.
Study variables. The recorded variables include: (1) preoperative (demographic, comorbidity, American Society of Anesthesiology [ASA] scale, bilirubin and hemoglobin); (2) intraoperative (venous resection, operative time and transfusion); (3) postoperative (complications according to the Clavien-Dindo classification18).
Pancreatic fistula19, post-pancreatectomy hemorrhage20 and delayed gastric emptying were diagnosed according to the definitions of the International Study Group of Pancreatic Surgery (ISGPS)21.
Perioperative mortality was defined as deaths occurred during hospitalization or in the first 60 days after surgery. Hospital stay and the percentage of readmissions were also analyzed. Likewise, we recorded the number of invaded/resected nodes, resection margin involvement and tumor size. Postoperative follow-up was performed to estimate overall and disease-free survival 1, 3, and 5 years after surgery.
Statistical analysisQuantitative variables were expressed as means and standard deviation, then compared with the Student’s t. Categorical variables were expressed in absolute numbers and percentages and compared with the Fischer or Chi-square test. Morbidity and mortality were analyzed with a bivariate analysis, estimating incidence ratios between the 2 age groups (relative risks, or RR) with their corresponding 95% confidence intervals (95%CI). The overall survival curves were constructed with the Kaplan-Meier estimator. The alpha level of statistical significance was set at 0.05, and the statistical analysis was performed with the SPSS program, version 24.
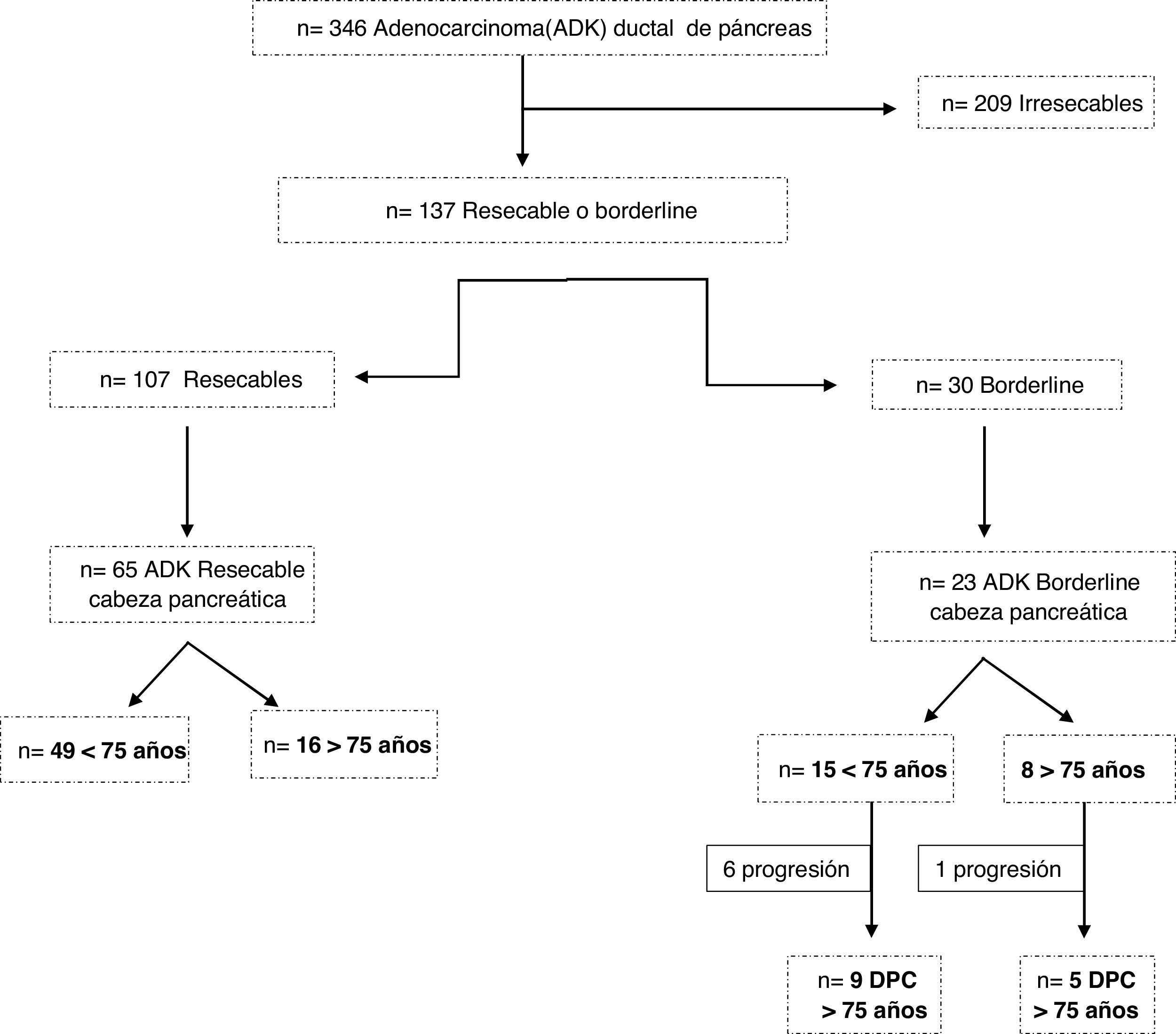
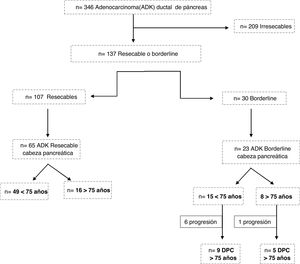
ResultsBetween 2007 and 2018, 309 patients were diagnosed with pancreatic ductal adenocarcinoma. Of these, 209 were considered unresectable tumors, 107 resectable and 30 borderline (Fig. 1). Surgery was ruled out in 5 patients ≥75 years, 4 due to age and comorbidity (3 resectable and one borderline) and one borderline due to tumor progression.
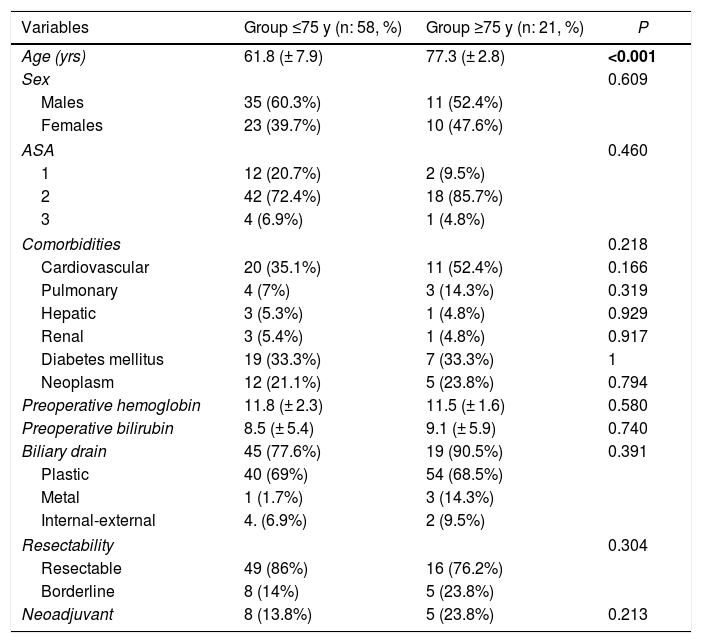
A total of 79 patients were included and classified into 2 groups: <75 years (58 patients; 73%) and ≥75 years (21 patients; 27%); within the latter, 6 patients were ≥80 years old (23.9%). Mean age was 66 ± 9.7 years (62 ± 7.9 years in the <75 years group and 77 ± 2.8 in the ≥75 years group). Elderly patients presented a higher ASA and more comorbidity, although with no differences (Table 1). Patients with borderline neoplasms received neoadjuvant treatment with gemcitabine-oxaliplatin, abraxane-gemcitabine, or folfirinox, depending on the date of inclusion and functional status, with no significant differences between the 2 groups.
Preoperative characteristics.
| Variables | Group ≤75 y (n: 58, %) | Group ≥75 y (n: 21, %) | P |
|---|---|---|---|
| Age (yrs) | 61.8 (± 7.9) | 77.3 (± 2.8) | <0.001 |
| Sex | 0.609 | ||
| Males | 35 (60.3%) | 11 (52.4%) | |
| Females | 23 (39.7%) | 10 (47.6%) | |
| ASA | 0.460 | ||
| 1 | 12 (20.7%) | 2 (9.5%) | |
| 2 | 42 (72.4%) | 18 (85.7%) | |
| 3 | 4 (6.9%) | 1 (4.8%) | |
| Comorbidities | 0.218 | ||
| Cardiovascular | 20 (35.1%) | 11 (52.4%) | 0.166 |
| Pulmonary | 4 (7%) | 3 (14.3%) | 0.319 |
| Hepatic | 3 (5.3%) | 1 (4.8%) | 0.929 |
| Renal | 3 (5.4%) | 1 (4.8%) | 0.917 |
| Diabetes mellitus | 19 (33.3%) | 7 (33.3%) | 1 |
| Neoplasm | 12 (21.1%) | 5 (23.8%) | 0.794 |
| Preoperative hemoglobin | 11.8 (± 2.3) | 11.5 (± 1.6) | 0.580 |
| Preoperative bilirubin | 8.5 (± 5.4) | 9.1 (± 5.9) | 0.740 |
| Biliary drain | 45 (77.6%) | 19 (90.5%) | 0.391 |
| Plastic | 40 (69%) | 54 (68.5%) | |
| Metal | 1 (1.7%) | 3 (14.3%) | |
| Internal-external | 4. (6.9%) | 2 (9.5%) | |
| Resectability | 0.304 | ||
| Resectable | 49 (86%) | 16 (76.2%) | |
| Borderline | 8 (14%) | 5 (23.8%) | |
| Neoadjuvant | 8 (13.8%) | 5 (23.8%) | 0.213 |
Statistically significant values are in bold.
The overall transfusion rate was 52%. Patients ≥75 years of age had a higher intraoperative transfusion index (RR = 2.07; 95%CI: 1.02–4.21) (Table 2), with a mean of 1.06 ± 1.6 units of packed red blood cells. Postoperative transfusion was also higher in group B (68% vs 42.4%), with a mean of 0.33 ± 0.78 units vs 0.9 ± 1.74 units (P = .03) (Table 3). Likewise, more vascular resections were performed in elderly patients (26% vs 8.7%; P = .037) (Table 2) and in those with borderline neoplasms (61.5% vs resectable neoplasms 18.5%; P = .01).
Intraoperative and pathological results.
| Group ≤75 y (n: 58, %) | Group ≥75 y (n: 21, %) | P | |
|---|---|---|---|
| Venous resection | 12 (20.7%) | 8 (38.1%) | 0.037 |
| Segmental | 4 | 2 | |
| Lateral | 8 | 6 | |
| Surgical time (h) | 6.2 ± 0.7 | 6.4 ± 0.9 | 0.271 |
| Intraoperative transfusion | 10 (17.2%) | 6 (30%) | 0.223 |
| Number of intraoperative units | 0.33 ± 0.78 | 0.9 ± 1.74 | 0.029 |
| Histological invasion of the wall SMV/P | 11 (90%) | 4 (60%) | 0.084 |
| Resectability | 0.873 | ||
| R0 | 46 (79.3%) | 17 (81%) | |
| R1 | 12 (20.7%) | 4 (19%) | |
| Tumor grade | 5 (25%) | 0.741 | |
| G1 | 14 (24.6%) | 12 (60%) | |
| G2 | 30 (52.6%) | 3 (15%) | |
| G3 | 13 (22.8%) | ||
| Affected lymph nodes | 2.6 ± 2.4 | 2.48 ± 3.06 | 0.868 |
| Resected lymph nodes | 19.2 ± 9.3 | 19.8 ± 9.3 | 0.809 |
| Size | 2.85 ± 0.86 | 4.13 ± 4.86 | 0.057 |
| TNM | 0.638 | ||
| T1N0 | 2(3.4%) | 0 | |
| T1N1 | 1 (1.7%) | 0 | |
| T2N0 | 0 | 1 (4.8%) | |
| T2N1 | 5 (8.6%) | 2 (9.5%) | |
| T3N0 | 5 (8.6% | 3 (14.2%) | |
| T3N1 | 42 (72.3%) | 13 (61.9%) | |
| T4N1 | 1 (1.7%) | 0 | |
| Tumor stage | 0.831 | ||
| I | 2(3.5%) | 1 (5%) | |
| IIA | 5 (8.8%) | 3 (15%) | |
| IIB | 48 (84.2%) | 15 (75%) | |
| III | 2 (3.5%) | 1 (5%) |
Statistically significant values are in bold.
Postoperative evolution.
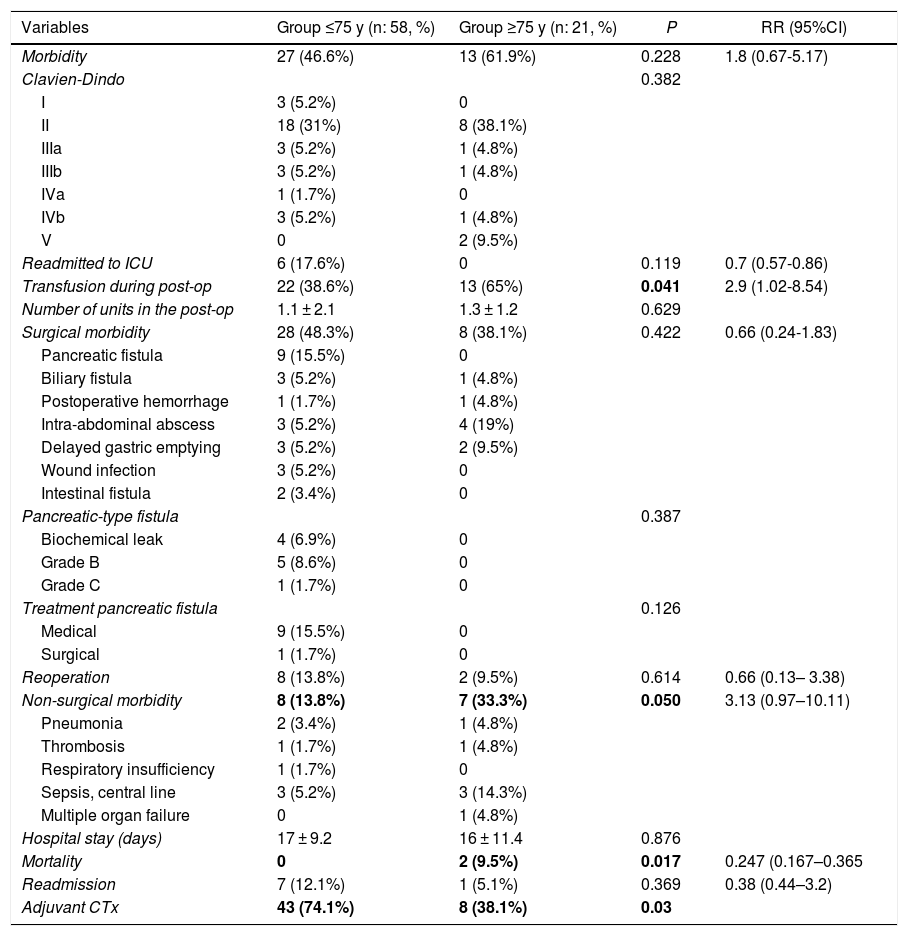
| Variables | Group ≤75 y (n: 58, %) | Group ≥75 y (n: 21, %) | P | RR (95%CI) |
|---|---|---|---|---|
| Morbidity | 27 (46.6%) | 13 (61.9%) | 0.228 | 1.8 (0.67-5.17) |
| Clavien-Dindo | 0.382 | |||
| I | 3 (5.2%) | 0 | ||
| II | 18 (31%) | 8 (38.1%) | ||
| IIIa | 3 (5.2%) | 1 (4.8%) | ||
| IIIb | 3 (5.2%) | 1 (4.8%) | ||
| IVa | 1 (1.7%) | 0 | ||
| IVb | 3 (5.2%) | 1 (4.8%) | ||
| V | 0 | 2 (9.5%) | ||
| Readmitted to ICU | 6 (17.6%) | 0 | 0.119 | 0.7 (0.57-0.86) |
| Transfusion during post-op | 22 (38.6%) | 13 (65%) | 0.041 | 2.9 (1.02-8.54) |
| Number of units in the post-op | 1.1 ± 2.1 | 1.3 ± 1.2 | 0.629 | |
| Surgical morbidity | 28 (48.3%) | 8 (38.1%) | 0.422 | 0.66 (0.24-1.83) |
| Pancreatic fistula | 9 (15.5%) | 0 | ||
| Biliary fistula | 3 (5.2%) | 1 (4.8%) | ||
| Postoperative hemorrhage | 1 (1.7%) | 1 (4.8%) | ||
| Intra-abdominal abscess | 3 (5.2%) | 4 (19%) | ||
| Delayed gastric emptying | 3 (5.2%) | 2 (9.5%) | ||
| Wound infection | 3 (5.2%) | 0 | ||
| Intestinal fistula | 2 (3.4%) | 0 | ||
| Pancreatic-type fistula | 0.387 | |||
| Biochemical leak | 4 (6.9%) | 0 | ||
| Grade B | 5 (8.6%) | 0 | ||
| Grade C | 1 (1.7%) | 0 | ||
| Treatment pancreatic fistula | 0.126 | |||
| Medical | 9 (15.5%) | 0 | ||
| Surgical | 1 (1.7%) | 0 | ||
| Reoperation | 8 (13.8%) | 2 (9.5%) | 0.614 | 0.66 (0.13– 3.38) |
| Non-surgical morbidity | 8 (13.8%) | 7 (33.3%) | 0.050 | 3.13 (0.97–10.11) |
| Pneumonia | 2 (3.4%) | 1 (4.8%) | ||
| Thrombosis | 1 (1.7%) | 1 (4.8%) | ||
| Respiratory insufficiency | 1 (1.7%) | 0 | ||
| Sepsis, central line | 3 (5.2%) | 3 (14.3%) | ||
| Multiple organ failure | 0 | 1 (4.8%) | ||
| Hospital stay (days) | 17 ± 9.2 | 16 ± 11.4 | 0.876 | |
| Mortality | 0 | 2 (9.5%) | 0.017 | 0.247 (0.167–0.365 |
| Readmission | 7 (12.1%) | 1 (5.1%) | 0.369 | 0.38 (0.44–3.2) |
| Adjuvant CTx | 43 (74.1%) | 8 (38.1%) | 0.03 |
Statistically significant values are in bold.
Overall morbidity was 50.6%. The elderly group had greater morbidity (61.9% vs 46.6%), although without differences (P = .228). Serious complications (III, IV) were similar in both groups (23.9% vs 23.3%). The most frequent surgical complication was pancreatic fistula (15.5%). Elderly patients presented more non-surgical complications (33.3%, P = .050) (RR = 3.13; 0.97–10.11), the most frequent being pneumonia and infection associated with the central line.
Postoperative mortality was 2.2% (2 patients), although it was higher in the ≥75 group (9% vs 0%; P = .017), with an RR of 0.247 (95%CI: 0.44–3.2) (Table 3). The causes of death were abdominal sepsis due to dehiscence of the hepaticojejunal anastomosis with multiple organ failure in one patient and early thrombosis of the polytetrafluoroethylene vein stent in one patient with segmental venous resection. In the multivariate analysis, age was not shown to be a risk factor for morbidity or mortality. However, mesenteric-portal venous resection was associated with higher mortality (P = .01), and perioperative transfusion was associated with higher overall morbidity (P = 0.06).
No significant differences were observed in the resection margins or in the number of affected/resected lymph nodes. Histological invasion of the venous wall was confirmed in 77.1% of patients (90% vs 60%; P = .084). Elderly patients received less adjuvant chemotherapy than patients <75 years; 38.1 vs 74.1% (P = .021).
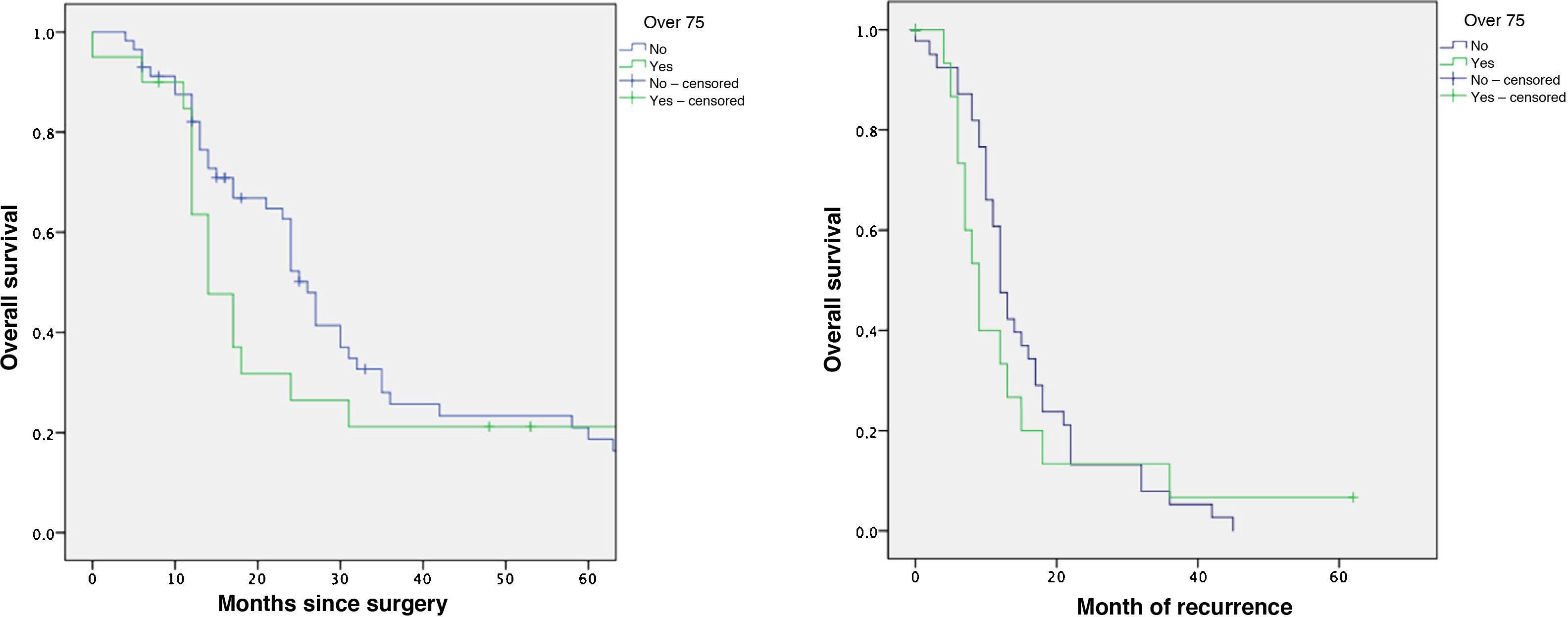
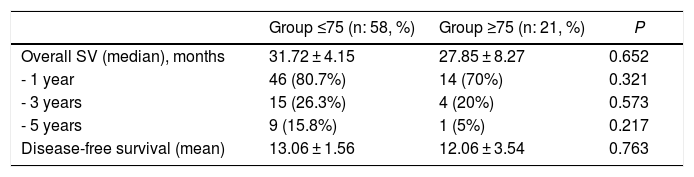
The mean overall survival of the series was 31 ± 32.7 months, with a median of 18 months. One-, 3- and 5-year survival rates were 78%, 25% and 13%, respectively (Fig. 2). Overall survival did not show significant differences, although it was significantly lower in the older group (Table 4). The median survival of the subgroup >80 years was 13.2 months. The multivariate analysis showed that the patients who received adjuvant chemotherapy had a longer survival (P = .01). Similarly, disease-free survival showed no differences (Table 4).
Overall survival and disease-free survival.
| Group ≤75 (n: 58, %) | Group ≥75 (n: 21, %) | P | |
|---|---|---|---|
| Overall SV (median), months | 31.72 ± 4.15 | 27.85 ± 8.27 | 0.652 |
| - 1 year | 46 (80.7%) | 14 (70%) | 0.321 |
| - 3 years | 15 (26.3%) | 4 (20%) | 0.573 |
| - 5 years | 9 (15.8%) | 1 (5%) | 0.217 |
| Disease-free survival (mean) | 13.06 ± 1.56 | 12.06 ± 3.54 | 0.763 |
As the elderly population grows, and both functional status and life expectancy improve, we are faced with an increasing demand to treat elderly patients with radiologically resectable pancreatic cancer. At the time of diagnosis, more than 50% of patients with pancreatic adenocarcinoma are over 70 years of age4,17. There is no unanimously accepted criterion to define the elderly population, so we set the cut-off point at 75 years, based on the literature and the World Health Organization classification10,12,17–19. In the present series, 27% of the patients were over 75 years of age, and 23% of them were in their eighties. In the series consulted, the geriatric population was less than 10%5,10, and few of them described patients older than 80 years.
Although the mortality rate of PD has decreased to below 5%, postoperative morbidity remains high, which is why the indication of PD in elderly patients is controversial10,11,14,15, given that older patients have less functional reserve4,20,21. In the study by Barbas et al.6, patients with a cardiovascular history had a higher postoperative mortality. In our series, patients >75 years presented similar ASA and comorbidity, indicating rigorous preoperative selection. In 39.2% of the patients, preoperative hemoglobin was <12 g. Thus, the treatment of preoperative anemia can play an important role in preoperative prehabilitation programs4,21. Since 2015, we have applied an ERAS (Enhanced Recovery After Surgery) protocol based on the results published in a study at our hospital22.
In recent years, vascular resections have been proposed to achieve a higher rate of resectability with free margins. This involves greater surgical complexity and postoperative morbidity. However, authors such as Kanda et al.23 have not recorded more complications in this subgroup of patients and conclude that age itself is not a contraindication. Unlike other studies6,22,23, more venous resections were performed in our series in the older group and in borderline neoplasms, while histological infiltration of the venous wall was similar between the 2 groups, which is in line with other series24.
Postoperative morbidity. The morbidity and mortality of PD in geriatric patients presents contradictory results, both for4,5,17 and against9,20,21,25. Although mortality has decreased, complications remain between 40% and 70%4. In the meta-analysis by Tan et al., morbidity was higher in the elderly group, although pancreatic fistulae, postoperative hemorrhage, intra-abdominal abscesses and delayed gastric emptying were comparable in both groups, suggesting that these complications were independent of age4. The Renz et al group did not identify independent risk factors in the development of surgical complications, although they indicated that age, male sex and the presence of cardiovascular comorbidities were independent for non-surgical complications, especially respiratory4,5. In our series, patients ≥75 years did not show significant differences in overall morbidity, serious complications, reoperations, or hospital stay. However, more non-surgical complications were observed, particularly pneumonia and infection associated with the central line. In the multivariate analysis, perioperative transfusion was a risk factor for increased overall morbidity, but age and venous resection were not.
Postoperative mortality. It has been suggested that elderly patients have a lower functional reserve, which could determine higher mortality in this age group4,11,15. The study by Busquets et al showed that advanced age, the presence of medical complications, as well as serious surgical complications, such as hemoperitoneum or anastomotic leaks, were risk factors for postoperative mortality.24 Authors such as Shamali and Turrini also found significant differences in mortality between both groups11,25. However, the meta-analysis by Sukharamwala et al did not show significant differences5. None of these studies identified age as an independent risk factor. In our series, patients ≥75 years had a higher mortality (9% vs 0%) (P = .02). In the multivariate analysis, age was not shown to be a risk factor, and mesenteric-portal venous resection was associated with higher mortality (P = .01), although this could be a bias due to the small population size.
Attempts have been made to identify prognostic factors associated with survival. Turrini et al.25 have suggested that patients >70 years of age receive less adjuvant chemotherapy due to their lower functional reserve or postoperative complications, and this fact could be related to shorter survival. Sho et al.26 reported that only 30% of patients >80 years received adjuvant chemotherapy, with an overall survival of 16.6 months, compared to 23.2 months in younger patients. In their multivariate analysis, the only independent prognostic factor was completion of adjuvant chemotherapy. In contrast, the Lu et al group27 estimated a similar median survival in both age groups. In the study by Shamali et al.11, the invasion of the margins, the number of affected lymph nodes, the presence of lymphovascular invasion and vascular resections were factors with a worse prognosis, unlike age, which did not show significant differences. Barbas et al. also described no significant differences in overall survival, but they did identify resection margins, tumor differentiation, and the administration of adjuvant chemotherapy as prognostic factors6. In our study, elderly patients received significantly less adjuvant chemotherapy; however, overall and disease-free survival showed no significant differences. It should be noted that the subgroup of octogenarian patients had a median survival of 13 months, compared to the overall median of 18 months in the rest of the series. These results suggest that chronological age alone should not be considered a contraindication for this type of intervention.
Limitations. The main limitation of this study is the small size of the geriatric population. This small sample prevented performing subgroup analyses and estimating interactions between the variables studied.
Conclusions. PD is a challenge in elderly patients since it presents a higher transfusion rate, more non-surgical complications and higher postoperative mortality, especially when venous resection is necessary. However, the similarity in survival results in both groups indicates that, with careful patient selection as well as proper surgical technique and postoperative care, age alone should not be considered a contraindication to surgery.
Conflict of interestsThe authors declare having no conflict of interests.
Please cite this article as: Pineño-Flores C, Ambrona-Zafra D, Rodríguez-Pino JC, Soldevila-Verdeguer C, Palma-Zamora E, Molina-Romero FX, et al. Duodenopancreatectomía por adenocarcinoma ductal de páncreas en ancianos. ¿Podemos asumirla con seguridad? Cir Esp. 2022;100:125–132.