Distal pancreatectomy (DP) is currently well established as a minimally invasive surgery (MIS) procedure, using either a laparoscopic (LDP) or robotic (RDP) approach.
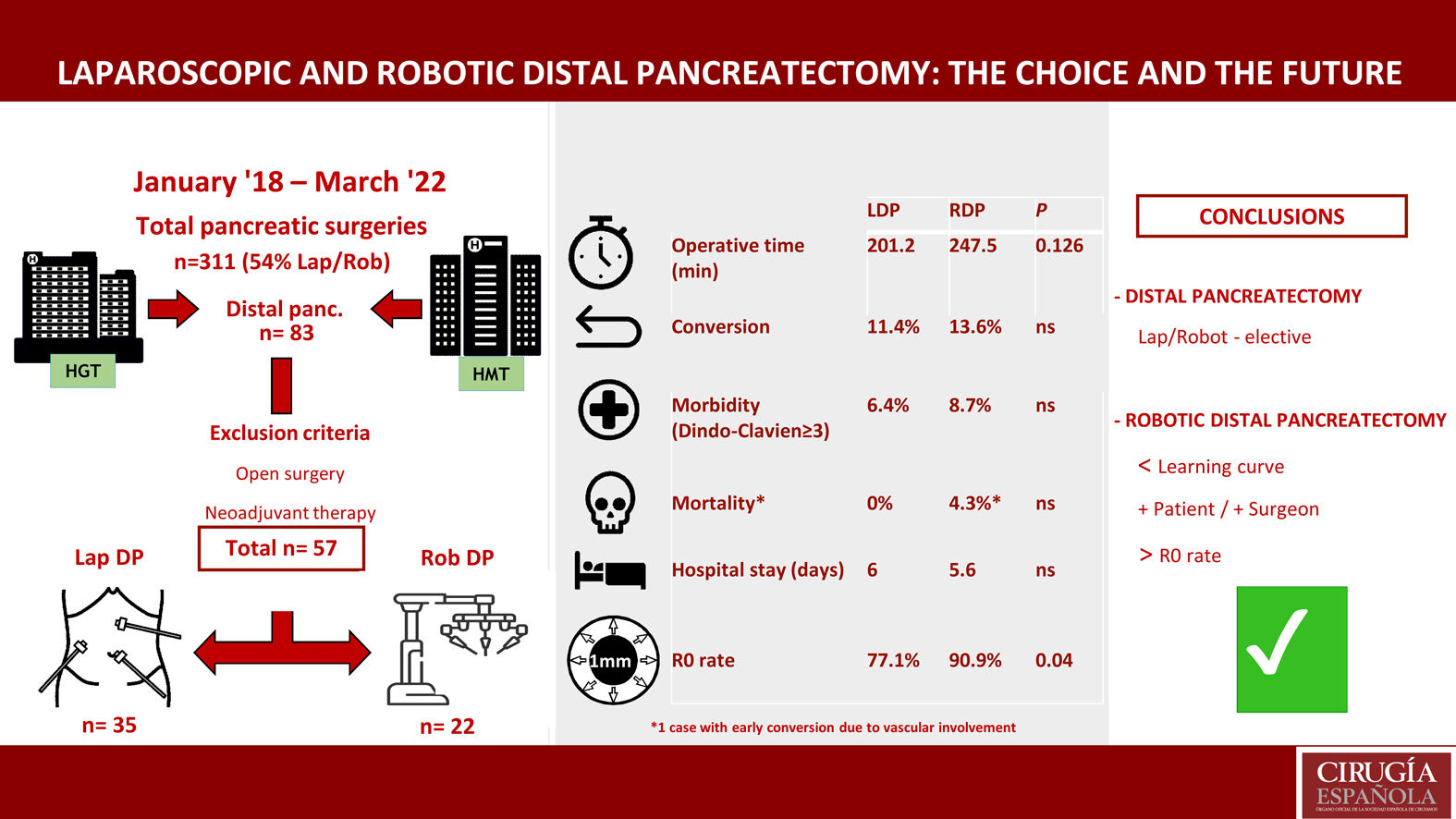
MethodsOut of 83 DP performed between January 2018 and March 2022, 57 cases (68.7%) were performed using MIS: 35 LDP and 22 RDP (da Vinci Xi). We have assessed the experience with the two techniques and analyzed the value of the robotic approach. Cases of conversion have been examined in detail.
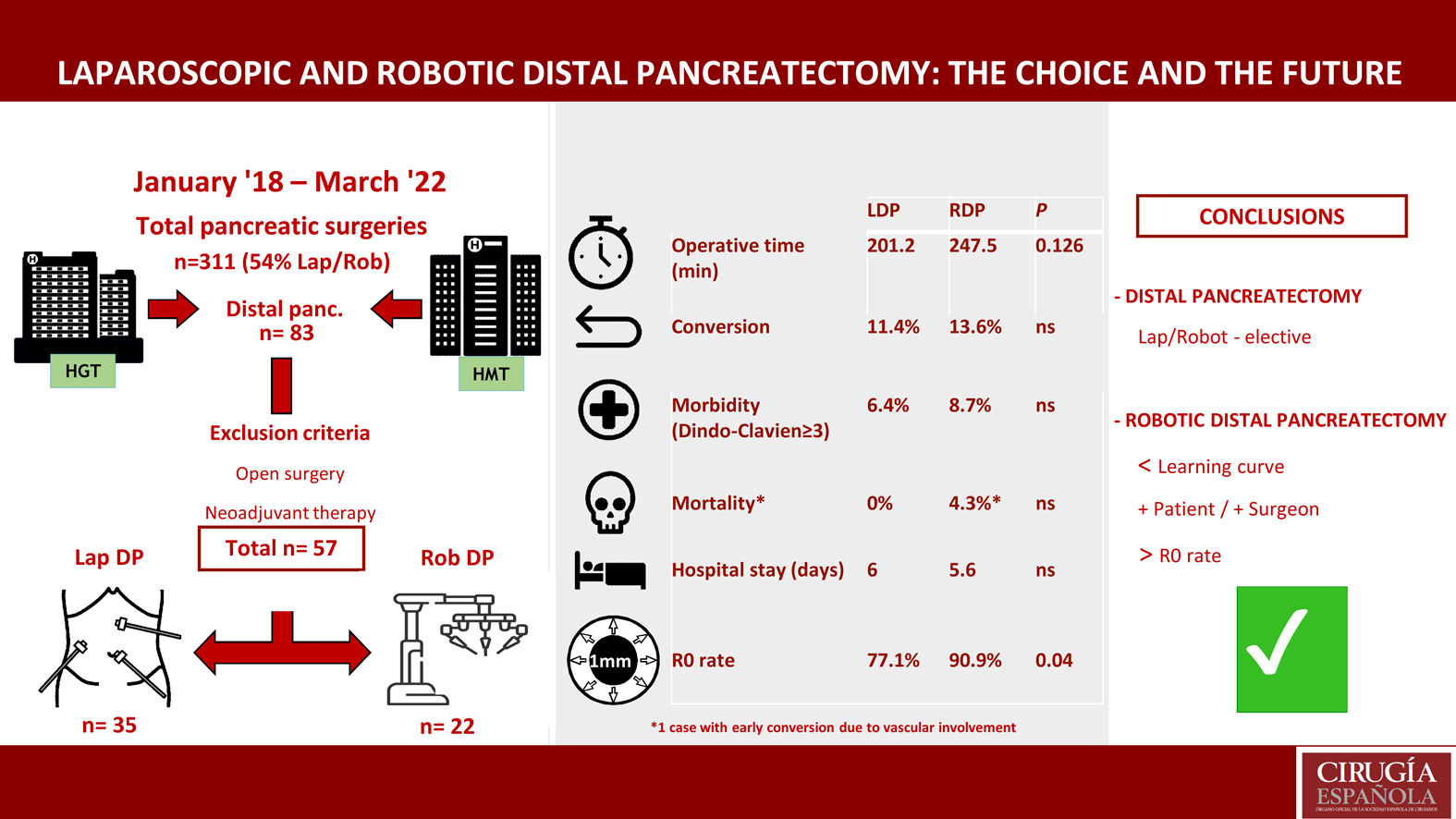
ResultsThe mean operative times for LDP and RDP were 201.2 (SD 47.8) and 247.54 (SD 35.8) minutes, respectively (P = NS). No differences were observed in length of hospital stay or conversion rate: 6 (5−34) vs. 5.6 (5−22) days, and 4 (11.4%) vs. 3 (13.6%) cases, respectively (P = NS). The readmission rate was 3/35 patients (11.4%) treated with LDP and 6/22 (27.3%) cases of RDP (P = NS).
There were no differences in morbidity (Dindo-Clavien ≥ III) between the two groups. Mortality was one case in the robotic group (a patient with early conversion due to vascular involvement). The rate of R0 resection was greater and statistically significant in the RDP group (77.1% vs. 90.9%) (P = .04).
ConclusionMinimally invasive distal pancreatectomy (MIDP) is a safe and feasible procedure in selected patients. Surgical planning and stepwise implementation based on prior experience help surgeons successfully perform technically demanding procedures. RDP could be the approach of choice in distal pancreatectomy, and it is not inferior to LDP.
La pancreatectomía distal (PD) mínimamente invasiva (MIS) está actualmente bien establecida, ya sea mediante técnica laparoscópica (PDL) o robótica (PDR).
MétodosDe 83 PD realizadas entre enero de 2018 y marzo de 2022 se realizaron 57 casos (68,7%) mediante MIS, 35 PDL y 22 PDR (da Vinci Xi). Se evalúa la experiencia de ambos procedimientos y el valor del abordaje robótico. Se analizan en detalle los casos de conversión.
ResultadosEl tiempo quirúrgico medio en las PDL y PDR fue de 201,2 (DE 47,8) y 247,54 (DE 35,8) minutos, p = ns. No se observaron diferencias en estancia hospitalaria ni en tasa de conversión, 6 (5−34) vs. 5,6 (5−22) días y 4 (11,4%) vs. 3 (13,6%) casos, respectivamente, p = ns. La tasa de reingresos fue de 3/35 (11,4%) y 6/22 (27,3%) casos, PDL vs. PDR respectivamente, p = ns.
No existieron diferencias en morbilidad (Dindo-Clavien ≥ III) entre ambos grupos. La mortalidad fue de un caso en el grupo robótico (un paciente con conversión precoz por afectación vascular). La tasa de resecciones R0 fue mayor en el grupo robótico (77,1% vs. 90,9%) alcanzando la significación estadística, p = 0.04.
ConclusiónLa PDMIS es un procedimiento seguro y factible en pacientes seleccionados. Una planificación quirúrgica y la implementación escalonada basada en la experiencia previa ayudan a afrontar procedimientos técnicamente exigentes. Se sugiere que la PDR podría ser el abordaje de elección en la pancreatectomía corporocaudal, no siendo inferior a la PDL.
The first laparoscopic distal pancreatectomy was performed in 1994.1 Since then, the spread of minimally invasive surgery (MIS) of the pancreas has been slow and irregular.2–3 In recent years, robotic surgery has emerged, although few hospitals in our setting have adopted this technique.4–6
It has been demonstrated that minimally invasive pancreatic procedures, especially laparoscopic distal pancreatectomy (LDP) as well as robotic distal pancreatectomy (RDP), provide better and faster postoperative recovery while sustaining the oncological results achieved in open surgery.7,8 In 2019, the international consensus accepted that both techniques can be used indistinctly with a level of evidence 1B. However, in cases of malignant pathology (adenocarcinoma), the experts and 95% of the “audience” agreed that it is necessary to increase the level of evidence.1,9,10
The volume of patients treated at a hospital greatly affects the results, and the total numbers of pancreatic resections and pancreatic MIS9 are considered relevant factors. What is more controversial is whether experience in laparoscopic surgery improves learning in robotic surgery.11
Although series conducted at medical centers with high volumes of robotic procedures and multicenter studies have obtained promising results, their data are not conclusive.12,13 Meanwhile, the 3 randomized controlled trials currently underway have still not published any definitive results.14–16
At the Miami consensus meeting, it was stated that there is no clear evidence of the superiority of RDP over LDP. Instead, the approach to use should be determined by the training and experience of the surgeons and resources available at each medical center.7
This study analyzes a consecutive series of patients who underwent minimally invasive distal pancreatectomy (LDP and RDP), and the short-term results are presented. All procedures were performed in the same time period and by the same surgical group certified in robotic surgery. The study objective was to evaluate the feasibility, safety and oncological results of both techniques, as well as the postoperative morbidity and mortality rates. The value of robotic surgery in distal pancreatectomy will also be discussed.
MethodsStudy designWe have conducted a prospective, non-randomized study at 2 tertiary hospitals in the Barcelona metropolitan area (Hospital Universitari Germans Trias i Pujol and Hospital Universitari Mútua de Terrassa), with a combined reference population of 1 200 000 inhabitants. We analyzed the results of distal pancreatectomies (DP) performed with the laparoscopic and robotic approaches. In the RDP cases, the da Vinci Xi robotic system (Intuitive Surgical, Sunnyvale, CA, USA) was used in all cases.
Inclusion and exclusion criteriaThe study included all patients who had undergone elective minimally-invasive DP for either benign or malignant disease. The exclusion criteria were: previous treatment with neoadjuvant therapy, and presence of tumors of the neck/body of the pancreas with possible vascular involvement, as these procedures were performed using an open approach.
Study populationFrom January 2018 to April 2022, 311 pancreatic surgeries were performed at these medical centers, 168 of which (54%) were performed using MIS. Out of the 83 DP carried out in this period, 57 cases were analyzed (35 LDP and 22 RDP).
Main variablesThe main variables studied were: operative time, hospital stay, conversion rate, severe morbidity (Dindo-Clavien ≥ III17), cumulative morbidity estimated using the CCI18 (comprehensive complication index) scale, and 30-day/90-day associated mortality rates.
For this study, we have followed the consensus document on the standardization of terminology in minimally invasive pancreatic surgery.19 Conversion was defined as the change in surgical technique, either from robotic to laparoscopic surgery or from laparoscopy to laparotomy to continue or complete the procedure.
We have avoided using the terms “purely robotic” or “combined robotic”, as these cases were considered robotic procedures.
Secondary variablesSecondary variables included: pancreatic fistulae, postoperative bleeding, resection margin, and 30-day/90-day readmission rates.
Pancreatic fistulae and postoperative bleeding were defined by the definitions established by the International Study Group of Pancreatic Surgery (ISGPS).20,21
R0 was defined by a distance from the tumor margin >1 mm, R1 if the distance from the tumor margin was <1 mm, and R2 if there was a macroscopic tumor in the resection margin.22
Statistical analysisThis was carried out following an intention-to-treat analysis using SPSS software (version 25). The distribution of normality was studied using the Kolmogorov-Smirnov test, and the variables were analyzed using the Mann-Whitney U and chi-squared tests. P values <.05 were considered statistically significant.
ResultsDuring the study period, 83 distal pancreatectomies were performed: 26 patients were treated with laparotomy, and the remaining 57 with a minimally invasive approach (35 LDP and 22 RDP).
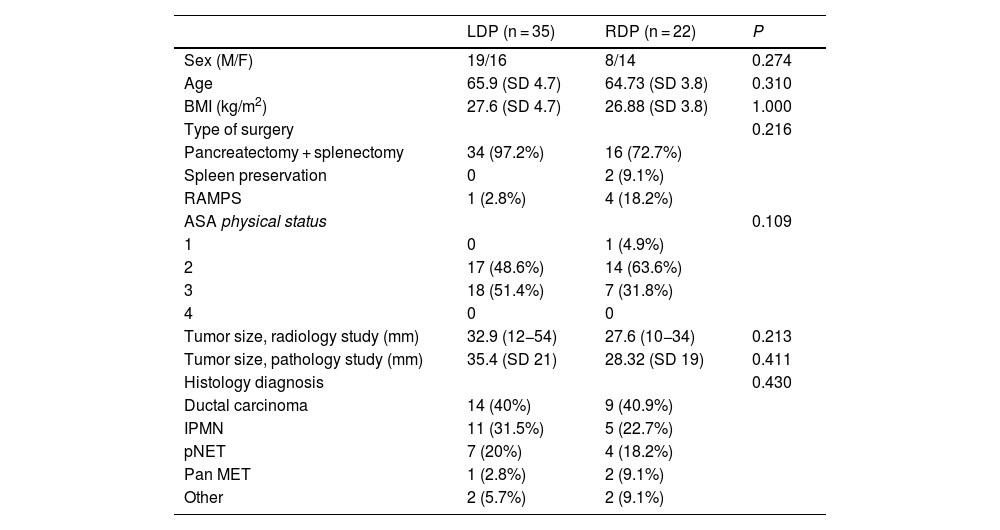
Patient characteristicsThere were no significant differences between the 2 groups for age, sex, BMI or ASA. In the laparoscopic group, 48.6% (n = 17) of the patients were ASA 2, and 51.4% (n = 18) were ASA 3. In the robotic group, 4.6% (n = 1) were ASA 1, 63.6% (n = 14) ASA 2, and 31.8% (n = 7) ASA 3.
The spleen was preserved in 2 cases of RDP.
The diagnosis of ductal carcinoma was 40% in LDP and 40.9% in RDP. The second most frequent diagnosis was IPMN (31.5% and 21.7%, respectively) (Table 1).
Baseline characteristics and demographic variables.
| LDP (n = 35) | RDP (n = 22) | P | |
|---|---|---|---|
| Sex (M/F) | 19/16 | 8/14 | 0.274 |
| Age | 65.9 (SD 4.7) | 64.73 (SD 3.8) | 0.310 |
| BMI (kg/m2) | 27.6 (SD 4.7) | 26.88 (SD 3.8) | 1.000 |
| Type of surgery | 0.216 | ||
| Pancreatectomy + splenectomy | 34 (97.2%) | 16 (72.7%) | |
| Spleen preservation | 0 | 2 (9.1%) | |
| RAMPS | 1 (2.8%) | 4 (18.2%) | |
| ASA physical status | 0.109 | ||
| 1 | 0 | 1 (4.9%) | |
| 2 | 17 (48.6%) | 14 (63.6%) | |
| 3 | 18 (51.4%) | 7 (31.8%) | |
| 4 | 0 | 0 | |
| Tumor size, radiology study (mm) | 32.9 (12−54) | 27.6 (10−34) | 0.213 |
| Tumor size, pathology study (mm) | 35.4 (SD 21) | 28.32 (SD 19) | 0.411 |
| Histology diagnosis | 0.430 | ||
| Ductal carcinoma | 14 (40%) | 9 (40.9%) | |
| IPMN | 11 (31.5%) | 5 (22.7%) | |
| pNET | 7 (20%) | 4 (18.2%) | |
| Pan MET | 1 (2.8%) | 2 (9.1%) | |
| Other | 2 (5.7%) | 2 (9.1%) |
LDP, laparoscopic distal pancreatectomy; RDP, robotic distal pancreatectomy; M/F, Males/Females; BMI, body mass index; ASA, American Society of Anesthesiologists; IPMN, intraductal papillary mucinous neoplasm; pNET, pancreatic neuroendocrine tumor; Pan MET, pancreatic metastasis; SD, standard deviation (interquartile range and percentages).
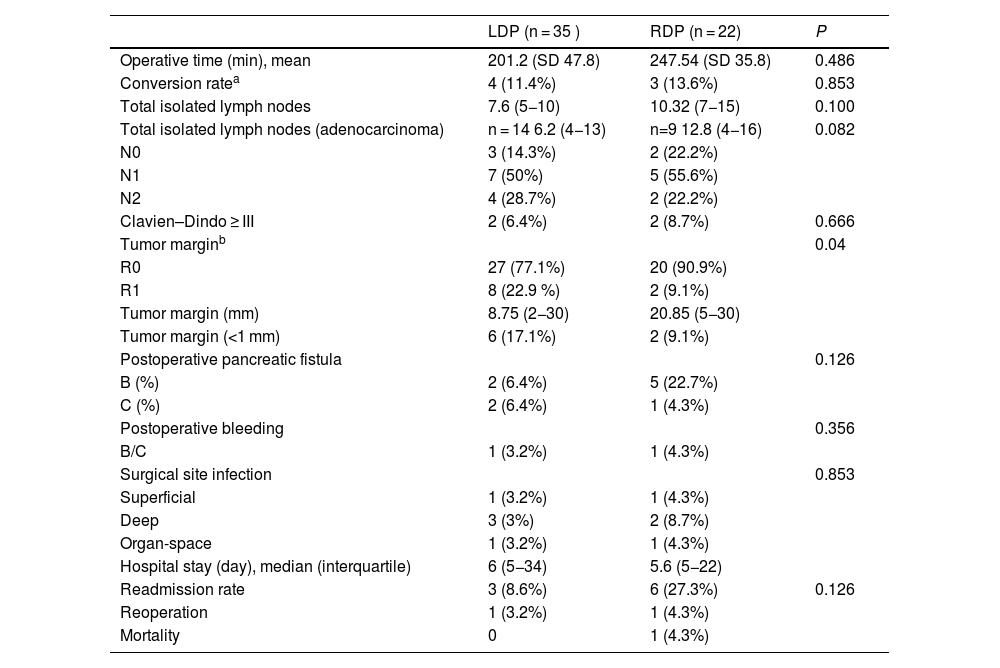
No significant differences were found between the operating times of the 2 surgical approaches. Mean operating times were 201.2 min in LDP and 247.54 min in RDP.
Mean hospital stay was 6 days (5−34) in the LDP group and 5.6 days (5−22) in the RDP group, with no significant differences.
Conversion to open surgery was necessary in 4 cases (11.4%) in the LDP group: 3 cases due to lack of progression, and one case due to difficult mobilization of the specimen (60-mm tumor). Three cases (13.6%) were converted in the RDP group: 2 because the portal-splenic confluence was encompassed, and one case due to involvement of the common hepatic artery.
There were no conversions from robotic to laparoscopic procedures, urgent conversions, or planned conversions. In all cases, the decision to convert was made due to difficult dissection and/or non-progression with risk of serious complications.
Morbidity was similar between the groups, and 2 patients in each group presented a significant complication in the Dindo–Clavien classification. The median CCI complication rate was 8.9 (0–20.6) for the LDP group and 20.9 (0–26.2) for RDP, with no statistical differences.
One patient from each group required reoperation: in the RDP group due to undetected gastric perforation, and for hemorrhage in the LDP group.
One patient from the RDP group with comorbidity required early conversion to open surgery due to extensive vascular involvement. This patient died during the immediate postoperative period due to pseudomonas-related pneumonia and multiple organ failure (Table 2).
Result for main and secondary variables.
| LDP (n = 35 ) | RDP (n = 22) | P | |
|---|---|---|---|
| Operative time (min), mean | 201.2 (SD 47.8) | 247.54 (SD 35.8) | 0.486 |
| Conversion ratea | 4 (11.4%) | 3 (13.6%) | 0.853 |
| Total isolated lymph nodes | 7.6 (5−10) | 10.32 (7−15) | 0.100 |
| Total isolated lymph nodes (adenocarcinoma) | n = 14 6.2 (4−13) | n=9 12.8 (4−16) | 0.082 |
| N0 | 3 (14.3%) | 2 (22.2%) | |
| N1 | 7 (50%) | 5 (55.6%) | |
| N2 | 4 (28.7%) | 2 (22.2%) | |
| Clavien–Dindo ≥ III | 2 (6.4%) | 2 (8.7%) | 0.666 |
| Tumor marginb | 0.04 | ||
| R0 | 27 (77.1%) | 20 (90.9%) | |
| R1 | 8 (22.9 %) | 2 (9.1%) | |
| Tumor margin (mm) | 8.75 (2−30) | 20.85 (5−30) | |
| Tumor margin (<1 mm) | 6 (17.1%) | 2 (9.1%) | |
| Postoperative pancreatic fistula | 0.126 | ||
| B (%) | 2 (6.4%) | 5 (22.7%) | |
| C (%) | 2 (6.4%) | 1 (4.3%) | |
| Postoperative bleeding | 0.356 | ||
| B/C | 1 (3.2%) | 1 (4.3%) | |
| Surgical site infection | 0.853 | ||
| Superficial | 1 (3.2%) | 1 (4.3%) | |
| Deep | 3 (3%) | 2 (8.7%) | |
| Organ-space | 1 (3.2%) | 1 (4.3%) | |
| Hospital stay (day), median (interquartile) | 6 (5−34) | 5.6 (5−22) | |
| Readmission rate | 3 (8.6%) | 6 (27.3%) | 0.126 |
| Reoperation | 1 (3.2%) | 1 (4.3%) | |
| Mortality | 0 | 1 (4.3%) |
LDP, laparoscopic distal pancreatectomy; RDP, robotic distal pancreatectomy; SD, standard deviation (interquartile range and percentages).
The robotic group obtained a higher rate of R0 resection (90.9%; n = 20) compared to the laparoscopic group (77.1%; n = 27), and this difference was statistically significant (P = .04).
The mean total number of isolated lymph nodes in the laparoscopic approach (7.6; range 5−10) was lower compared to RDP (10.3; range 7−15), which did not reach statistical significance.
The pancreatic fistula rate was 12.8% (n = 4) in the LDP patient group, specifically 2 grade B and 2 grade C fistulae. In the robotic group, the rate was 27% (n = 6), including 5 grade B fistulae B and only one grade C pancreatic fistula. No significant differences were found between the groups.
One patient in each group (3.2% in LDP and 4.3% in RDP) presented grade B or C postoperative hemorrhage.
The readmission rate was lower in the LDP group (8.6%, n = 3), including one case due to nosocomial pneumonia and 2 due to postoperative collection. One case required reoperation due to severe organ-space infection, and another required external drainage. All patients progressed favorably.
In the RDP group, 6 patients (27.3%) required readmission: 2 cases due to atelectasis/pneumonia, and 4 due to postoperative collections. Two of these cases were resolved with drainage and the remainder with antibiotic treatment (Table 2).
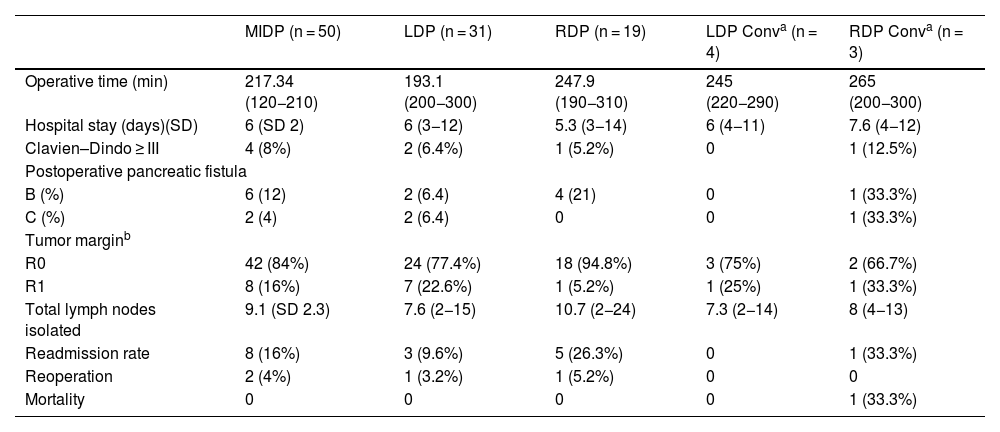
Analysis of converted casesMean operative time for all pancreatectomies without conversion was 217.3 min. In the converted laparoscopy group, mean operative time was 245 min, and in the converted robotic group it was 265 min.
Hospital stay was similar in all groups regardless of whether conversion was required or not. There were no significant differences in terms of postoperative complications between the various groups: laparoscopic without conversion, 2 patients (6.4%); robotic without conversion, 1 patient (6.2%); laparoscopic with conversion, no postoperative complications; and robotic with conversion, 1 patient (12.5%).
When we compared the groups with versus without conversion to open surgery, no differences were found in the incidence of pancreatic fistulae, readmission rate, or R0 rate (Table 3).
Comparative analysis between approach and conversion.
| MIDP (n = 50) | LDP (n = 31) | RDP (n = 19) | LDP Conva (n = 4) | RDP Conva (n = 3) | |
|---|---|---|---|---|---|
| Operative time (min) | 217.34 (120−210) | 193.1 (200−300) | 247.9 (190−310) | 245 (220−290) | 265 (200−300) |
| Hospital stay (days)(SD) | 6 (SD 2) | 6 (3−12) | 5.3 (3−14) | 6 (4−11) | 7.6 (4−12) |
| Clavien–Dindo ≥ III | 4 (8%) | 2 (6.4%) | 1 (5.2%) | 0 | 1 (12.5%) |
| Postoperative pancreatic fistula | |||||
| B (%) | 6 (12) | 2 (6.4) | 4 (21) | 0 | 1 (33.3%) |
| C (%) | 2 (4) | 2 (6.4) | 0 | 0 | 1 (33.3%) |
| Tumor marginb | |||||
| R0 | 42 (84%) | 24 (77.4%) | 18 (94.8%) | 3 (75%) | 2 (66.7%) |
| R1 | 8 (16%) | 7 (22.6%) | 1 (5.2%) | 1 (25%) | 1 (33.3%) |
| Total lymph nodes isolated | 9.1 (SD 2.3) | 7.6 (2−15) | 10.7 (2−24) | 7.3 (2−14) | 8 (4−13) |
| Readmission rate | 8 (16%) | 3 (9.6%) | 5 (26.3%) | 0 | 1 (33.3%) |
| Reoperation | 2 (4%) | 1 (3.2%) | 1 (5.2%) | 0 | 0 |
| Mortality | 0 | 0 | 0 | 0 | 1 (33.3%) |
MIDP, minimally invasive distal pancreatectomy; LDP, laparoscopic distal pancreatectomy; RDP, robotic distal pancreatectomy.
Several studies have shown significant advantages of laparoscopic distal pancreatectomy over the open approach.23–25 Two randomized controlled trials (RCT) have confirmed the results of previously published series. The first RCT compared both robotic and laparoscopic distal pancreatectomies with open surgery,8 demonstrating a difference in functional recovery that was statistically significant in favor of the minimally invasive approach (4 vs. 6 days of hospital stay). The second RCT compared LDP with open surgery,8 with a hospital stay of 5 vs. 6 days and a conversion rate of 3.4%. In our series, we have observed a hospital stay of 5–6 days in both groups, which is similar to those reports.
The longer operative time of LDP becomes considerably shorter as the learning curve is overcome, which takes between 10–37 cases according to published studies.26,27 In our series, LDP presents an operating time that is consistent with the literature, with low morbidity and adequate oncological results, and 41% of cases are operated on for adenocarcinoma.
An interesting publication analyzed 1807 patients who received chemotherapy after distal pancreatectomy (open vs. MIS), and minimally invasive DP (51 LDP/454 RDP) was associated with a higher rate of adjuvant chemotherapy.28 We believe this could be because minimally invasive surgery is associated with faster recovery due to a less aggressive procedure. This is relevant since the complete treatment for pancreatic cancer is surgery + chemotherapy, and increasing the number of cases with complete treatment could improve overall survival.
Robotic distal pancreatectomy adds to the advantages of laparoscopic surgery: better vision, greater precision in dissection, and vascular control. All these factors, as well as the similarity with the natural movements of the hands, results in a shorter learning curve. However, its use is still less frequent than the laparoscopic approach.29,30
In our experience, RDP obtains results similar to LDP, with very similar hospital stay and morbidity. Although the operating time was slightly longer, the R0 rate in cases of pancreatic adenocarcinoma was considerably higher in the robotic group, probably due to the previously described advantages, highlighting the ease and precision of dissection. This fact would lead to a higher rate of splenic preservation, which seems to be the trend in this series.
The conversion rates observed are similar between both procedures and are in accordance with the series mentioned: 11.4% for LDP and 13.6% for RDP. These data support the fact that robotic learning is faster and easier; while the laparoscopic surgery learning curve of the surgical group had been finalized, the patients included in the robotic group were the initial cases of the series. This indicates that robotic surgery is good for both the patient and the surgeon.
It is well known that unplanned conversion has higher morbidity and mortality rates.31,32 Although we do not consider the death in the RDP group of the series attributable to the robotic technique, early conversion to open surgery was due to extensive vascular involvement in a patient with significant comorbidities.
Regarding the economic cost of robotic surgery, various authors indicate an increased cost of more than 6000 euros when used for RDP.33,34 However, when postoperative recovery, hospital stay and quality of life are considered cumulatively, the cost of the robotic procedure is similar and even lower in the case of DP due to adenocarcinoma.30,35 We should emphasize that most of these data come from high-volume medical centers. Therefore, the current cost-effective analysis may not be applicable to lower-volume hospitals.
In our case, the incorporation of the robotic platform in hepatobiliary and pancreatic surgery was initiated within a previously established robotic surgery program, using all the available technology.
We currently believe that minimally invasive distal pancreatectomy is the approach of choice for both benign and malignant pathologies. Despite the lack of randomized studies, the lower surgical impact, shorter hospital stay, fewer complications, and better functional recovery would make it possible to administer adjuvant therapy in patients requiring it.
Although the benefits of RDP are not clear, and our series has not demonstrated any advantages over the laparoscopic approach, a shorter learning curve with greater oncological radicality extends the benefit for patients to the surgeon.
As put forth by a recent and interesting editorial by Azagra et al.,36 we agree that, “Moving from laparoscopic to robotic” surgery seems “to be a natural evolution,” in which the surgical equipment drags the surgeon along, while the standardization of the technique with that equipment becomes key to the process.
With greater experience and the development of robotic surgery skills, it is possible to increase the number of cases with preserved pancreatic parenchyma, without the need for extensive pancreatectomies. The ability of the robotic platform to incorporate artificial intelligence, 3D modeling and augmented reality will enhance its added value in the future.
Our experience in RDP allows us to affirm that the previous step of training in laparoscopic surgery is not essential and that the jump from open to robotic surgery is feasible.
In our opinion, and especially at the beginning of the learning curve, proper case selection is essential. This means starting with patients whose tumors are far from the neck of the pancreas and have no involvement of the posterior plane, later progressing to cases of splenic preservation37 and RAMPS.38,39
ConclusionsIn a selected group of patients, minimally invasive DP is safe and effective when performed by groups experienced in pancreatic surgery and minimally invasive surgery.
The added value provided by the robotic approach includes better vision, greater precision in dissection, and vascular control, which in our experience probably results in a shorter learning curve.
In this series, the robotic approach presents a similar hospital stay, with the same morbidity rate, and is oncologically adequate in cases of malignancy, with a higher R0 rate.
With more experience, RDP will likely be the quality standard. Future randomized multicenter studies are needed to confirm this hypothesis.