In multicenter studies, the protocolization of data is a critical phase that can generate biases.The objective is to analyze the concordance and reliability of the data obtained in a clinical multicenter study between the protocolization in the center of origin and the centralized protocolization of the data by a data -manager.
MethodsNational multicenter clinical study about an infrequent carcinoma. A double protocolization of the data is carried out: (a) center of origin; and (b) centralized by a data manager: The concordance between the data is analyzed for the global data and for the two groups of the project: (a) study group (Familiar carcinoma, 30 researchers protocolize); (b) control group (Sporadic carcinoma, 4 people protocolize). Interobserver variability is evaluated using Cohen's kappa coefficient.
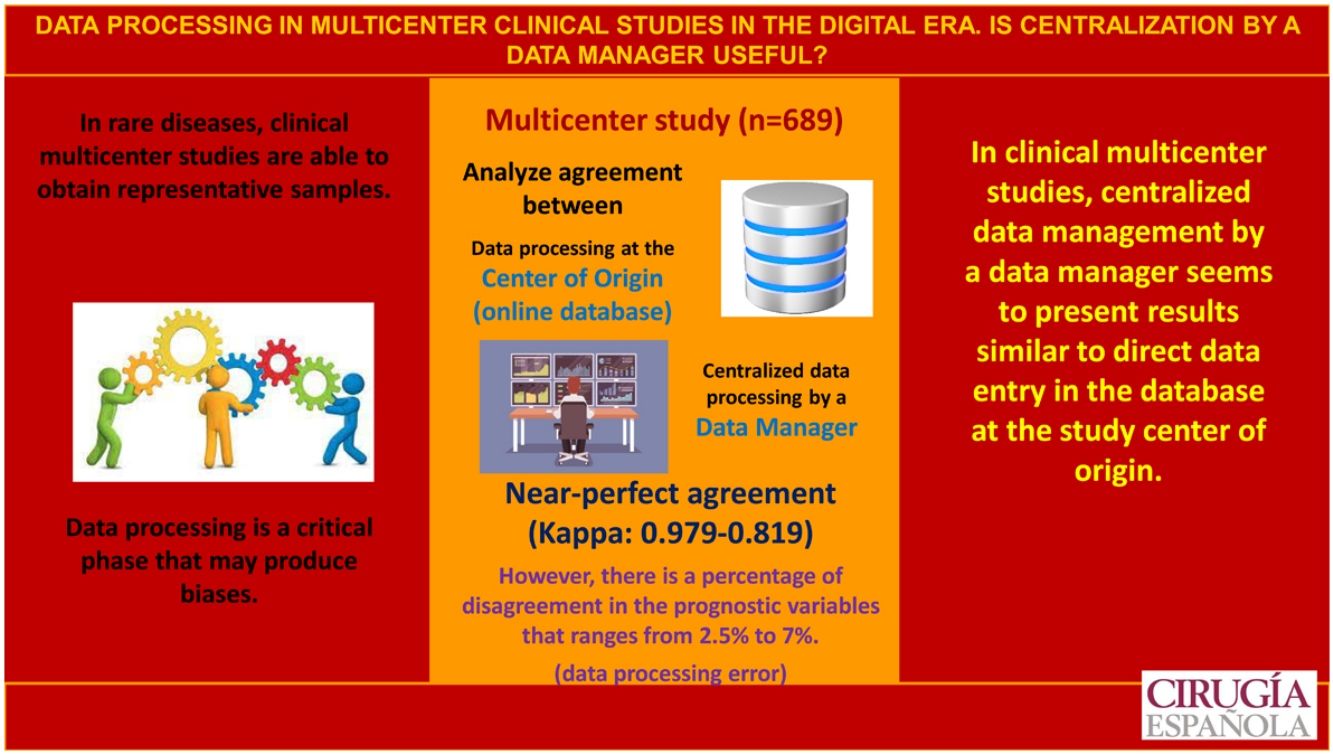
ResultsThe study includes a total of 689 patients with carcinoma, 252 in the study group and 437 in the control group. Regarding the concordance analysis of the tumor stage, 2.5% of disagreements were observed and the concordance between people who protocolize was near perfect (Kappa = 0.931). Regarding the evaluation of the recurrence risk, disagreements occurred in 7% of the cases and the concordance was near perfect (Kappa = 0.819). Regarding the sonography evaluation (TIRADS), the disagreements were 6.9% and the concordance was near perfect (Kappa = 0.922). Also, 4.6% of transcription errors were detected.
ConclusionsIn multicenter clinical studies, the centralized data protocolization o by a data-manager seems to present similar results to the direct protocolization in the database in the center of origin.
En los estudios multicéntricos la protocolización de los datos es una fase crítica que puede generar sesgos, sobre todo en estudios clínicos con presupuesto limitado. El objetivo es analizar la concordancia y cofiabilidad de los datos obtenidos en un estudio multicéntrico clínico entre la protocolización el centro de origen y la protocolización centralizada mediante un data-manager.
MétodoEstudio clínico multicéntrico de prevalencia nacional sobre un carcinoma familiar infrecuente, realizándose una doble protocolización de los datos: (a) en el centro de origen; y, (b) centralizada con un data-manager: La concordancia se analiza para el global de los datos y para los dos subgrupos del proyecto: (a) grupo a estudio (Carcinoma familiar. Protocolizan 30 investigadores); y (b) grupo control (Carcinoma esporádico. Protocolizan 4). Las diferencias interobservador se evalúan mediante el Indice de Kappa de Cohen.
ResultadosSe incluyen 689 pacientes, 252 del grupo a estudio y 437 del grupo control. Respecto al análisis de concordancia del estadio tumoral se han objetivado un 2,5% de discordancias, siendo alta la concordancia entre protocolizadores (Kappa = 0,931). Respecto a la valoración del riesgo de recidiva las discordancias fueron del 7% de los casos, siendo alta la concordancia (Kappa = 0,819). Respecto a la clasificación ecográfica TIRADS las discordancias son del 6,9% y la concordancia es alta (Kappa = 0,922). Se han detectado un 4,6% de errores de transcripción.
ConclusionesEn los estudios multicéntricos clínicos la protocolización centralizada de los datos por un data-manager parece presentar resultados similares a la protocolización directa en la base de datos en el centro de origen.
In rare pathologies, multicenter clinical studies are being promoted to achieve sufficiently large series over a relatively short period of time that would make it possible to draw representative conclusions.1,2 However, these studies also have drawbacks, particularly since patients are managed at different hospitals and with protocols that are not always the same, which generates a heterogeneity that may affect the results. Furthermore, remote work conducted by different researchers can lead to different biases.3
One step where biases can occur is data management.3 Currently, the trend is to generate an online cloud database that allows all study centers to manage data simultaneously. However, several researchers are involved in the data entry process, and this may lead to increased heterogeneity when compiling the database. In contrast, centralized data entry, generally by a data manager, entails greater expense and has not been shown to be beneficial.3
In most clinical trials, especially drug trials, electronic data collection is done directly, using a database and data capture systems to provide greater data integrity and shorten the study time. However, these systems are not easy to implement4 since they require prior installation by the research center, user training and patient participation — all of which come at a cost. Therefore, these systems are not very cost-effective for multicenter clinical projects,1,4 as their cost and the infrastructure required are not affordable. Furthermore, the collection of electronic data through a capture system is not free of risks, and its advantages can be lost when not used properly.5
What has become widespread in multicenter clinical projects (most without specific funding) is the use of online cloud databases for data processing. However, whatever potential biases or errors this online data collection system may entail has not been tested against a system of data entry by a data manager,6,7 especially when you consider that a reliable data storage system does not guarantee the reliability of data entry. Errors in data transcription have been reported, and fields near the end of the data entry page are associated with a higher number of errors. However, even in these instances, there are studies that indicate that control by a data manager can be considered an avoidable cost, while some authors suggest using an electronic control to monitor the reliability of data entry.6 Cost studies on the electronic data collection process in clinical trials have shown a reduction in collection costs of more than 50%, with savings ranging from 49 %–62 %, depending on the project.8
The aim of this study is to conduct a multicenter clinical study to analyze the agreement between the completion of the data collection protocol at the hospital of origin versus centralized data entry by a data manager in order to assess the reliability of the data.
MethodsStudy designWe have conducted a nation-wide multicenter study in Spain, which has been endorsed by the Spanish Association of Surgeons (Asociación Española de Cirujanos, or AEC). This study was the first main objective of our project. The study included patients with familial cancer of low incidence among the population.
We designed a specific data collection protocol for this pathology to collect socio-personal, clinical, therapeutic and follow-up variables. This protocol included an attached report specifying the data to be collected for each of the variables. In addition, contact information was provided to resolve any questions.
Data collectionThe data were collected at the center of origin by each of the researchers collaborating with the project, who complied with the project protocol. Said protocol may have been on paper or digitalized, but the data did not flow directly into the project database.
A double protocol was carried out.
- 1)
Protocol 1–Data entry at the center of origin: Data are registered as they are obtained, following the protocol at the center of origin.
- 2)
Protocol 2–Centralized data entry by a data manager: Data are registered in a centralized manner, by a single data manager, who is able to resolve any concerns about the analyzed variables with the study center of origin.
The agreement between the protocol conducted with the data from the center of origin and the centralized protocol using a data manager has been analyzed in 3 different groups:
- 1)
Group 1 - Overall study. Agreement was analyzed for the total sample under study.
- 2)
Group 2–Familial cancer group. Agreement in the familial cancer group was analyzed. In this group, 30 researchers participated in the collection of data at the study centers of origin, which were the different hospital units participating in the project.
- 3)
Group 3–Sporadic carcinoma control group. Agreement was analyzed in the control group of patients with sporadic carcinoma. In this group, 4 researchers participated in the data entry at the center of origin, as these data came from only 4 hospital units.
Most socio-personal, clinical and therapeutic variables were excluded from the agreement analysis, where the presence of discordance is exceptional, except for transcription error. Three groups of variables were analyzed:
- 1)
Prognostic classification according to the TNM system of the American Joint Committee on Cancer. Within this system, the following are analyzed:
- 2)
Size (T)
- 3)
Node involvement (N)
- 4)
Systemic involvement (M)
- 5)
TNM stage
- 6)
Assessment of the risk of recurrence according to the ATA (American Thyroid Association), which proposes 3 risk groups:
- 7)
Low risk
- 8)
Moderate risk
- 9)
High risk
- 10)
Ultrasound assessment and TI-RADS (Thyroid Imaging Reporting and Data System), according to the Korean TI-RADS classification.9 There are seven risk groups:
- 11)
TI-RADS 1
- 12)
TI-RADS 2
- 13)
TI-RADS 3
- 14)
TI-RADS 4a
- 15)
TI-RADS 4b
- 16)
TI-RADS 4c
- 17)
TI-RADS 5
Lastly, transcription errors detected during the data entry protocol process were assessed.
Statistical analysisThe statistical analysis was performed with a software package (SPSS, version 19.0 for Windows, SPSS, Chicago, Illinois). Interobserver differences were evaluated using Cohen's Kappa Index. The agreement coefficient has a value that ranges between 0 and 1, and its interpretation corresponds with the following ranges:
- •
0 No agreement.
- -
0.01–0.19 Very low agreement.
- -
0.20 – 0.39 Low agreement.
- -
0.40 – 0.59 Moderate agreement.
- -
0.60 – 0.79 Good agreement.
- -
0.80 – 0.99 High agreement.
- •
Perfect agreement.
P-values <.05 were considered statistically significant. There was agreement among the protocol data processers only if the correlation coefficient was greater than 0.00, with or without statistical significance.
For the comparison of groups 2 and 3, the Student’s t test was applied. Fisher’s exact test was applied when the contingency tables had cells with an expected frequency <5.
ResultsGeneral project characteristicsThe study included a total of 689 patients with cancer, divided between the familial cancer study group (n = 252) and the sporadic cancer control group (n = 437).
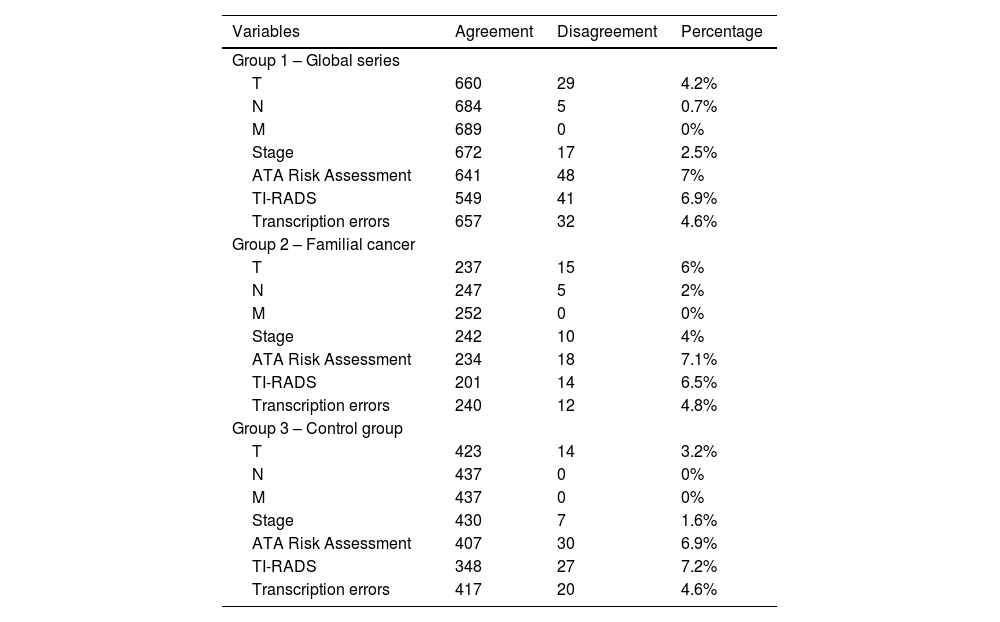
Agreement analysis regarding the prognostic classification of the TNM system1) Assessment of the T component of the TNM systemThe data management protocol for the T component of the TNM system presented a disagreement rate of 4.2% (n = 29). In the familial carcinoma group 2, with 30 data entry researchers, the disagreement rate reached 6% (n = 15). Meanwhile, in the sporadic carcinoma control group 3, with 4 protocol data entry researchers, discrepancies reached 3.2% (n = 14) (Table 1). These differences were not statistically significant (P = 0.125).
Percentage of disagreement between the initial assessment conducted at the hospital of origin and the final assessment centralized by a data manager.
| Variables | Agreement | Disagreement | Percentage |
|---|---|---|---|
| Group 1 – Global series | |||
| T | 660 | 29 | 4.2% |
| N | 684 | 5 | 0.7% |
| M | 689 | 0 | 0% |
| Stage | 672 | 17 | 2.5% |
| ATA Risk Assessment | 641 | 48 | 7% |
| TI-RADS | 549 | 41 | 6.9% |
| Transcription errors | 657 | 32 | 4.6% |
| Group 2 – Familial cancer | |||
| T | 237 | 15 | 6% |
| N | 247 | 5 | 2% |
| M | 252 | 0 | 0% |
| Stage | 242 | 10 | 4% |
| ATA Risk Assessment | 234 | 18 | 7.1% |
| TI-RADS | 201 | 14 | 6.5% |
| Transcription errors | 240 | 12 | 4.8% |
| Group 3 – Control group | |||
| T | 423 | 14 | 3.2% |
| N | 437 | 0 | 0% |
| M | 437 | 0 | 0% |
| Stage | 430 | 7 | 1.6% |
| ATA Risk Assessment | 407 | 30 | 6.9% |
| TI-RADS | 348 | 27 | 7.2% |
| Transcription errors | 417 | 20 | 4.6% |
Both from a global level (Kappa = 0.939) as well as in the familial cancer group 2 (Kappa = 0.920) and in control group 3 (Kappa = 0.950), the agreement among the data entry researchers was high (Table 2).
Agreement between the initial assessment made at the hospital of origin and the final assessment centralized by a data manager on the assessment of the T component (size) of the TNM system.
| Initial assessment at the Center of Origin | Centralized assessment by a Data Manager | |||||
|---|---|---|---|---|---|---|
| T1a | T1b | T2 | T3 | T4 | Total | |
| Group 1 – Overall seriesKappa coefficient for inter-rater reliability 0,939P <.0001 | ||||||
| T1a | 306 | 3 | 0 | 5 | 0 | 314 |
| T1b | 7 | 144 | 6 | 1 | 0 | 158 |
| T2 | 0 | 2 | 99 | 1 | 0 | 102 |
| T3 | 0 | 0 | 2 | 105 | 0 | 107 |
| T4 | 0 | 0 | 2 | 0 | 6 | 8 |
| Total | 313 | 149 | 109 | 112 | 6 | 689 |
| Group 2 – Familial cancerKappa coefficient for inter-rater reliability 0,920P <.0001 | ||||||
| T1a | 74 | 3 | 0 | 3 | 0 | 80 |
| T1b | 4 | 65 | 1 | 0 | 0 | 70 |
| T2 | 0 | 2 | 48 | 0 | 0 | 50 |
| T3 | 0 | 0 | 2 | 48 | 0 | 50 |
| T4 | 0 | 0 | 0 | 0 | 2 | 2 |
| Total | 78 | 70 | 51 | 51 | 2 | 252 |
| Group 3 – Control groupKappa coefficient for inter-rater reliability 0,950P <.0001 | ||||||
| T1a | 232 | 0 | 0 | 2 | 0 | 234 |
| T1b | 3 | 79 | 5 | 1 | 0 | 88 |
| T2 | 0 | 0 | 51 | 1 | 0 | 52 |
| T3 | 0 | 0 | 0 | 57 | 0 | 57 |
| T4 | 0 | 0 | 2 | 0 | 4 | 6 |
| Total | 235 | 79 | 58 | 61 | 4 | 437 |
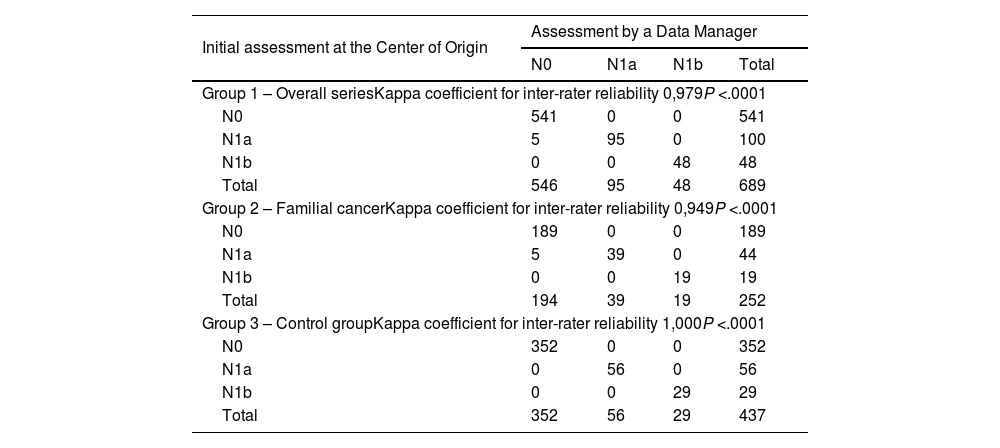
Data entries for the N component (lymph node involvement) of the TNM system presented a disagreement rate of 0.7% (n = 5), all of which were from the familial cancer group 2 (2%; n = 5) (P = .003) (Table 1).
Both globally (Kappa = 0.979) as well as in the familial cancer group 2 (Kappa = 0.949), agreement among the protocol data entry operators was high, and agreement was perfect in control group 3 (Kappa = 1.000) (Table 3).
Agreement between the initial assessment at the study center of origin and the centralized final assessment by a data manager regarding the N component (lymph node involvement) of the TNM system.
| Initial assessment at the Center of Origin | Assessment by a Data Manager | |||
|---|---|---|---|---|
| N0 | N1a | N1b | Total | |
| Group 1 – Overall seriesKappa coefficient for inter-rater reliability 0,979P <.0001 | ||||
| N0 | 541 | 0 | 0 | 541 |
| N1a | 5 | 95 | 0 | 100 |
| N1b | 0 | 0 | 48 | 48 |
| Total | 546 | 95 | 48 | 689 |
| Group 2 – Familial cancerKappa coefficient for inter-rater reliability 0,949P <.0001 | ||||
| N0 | 189 | 0 | 0 | 189 |
| N1a | 5 | 39 | 0 | 44 |
| N1b | 0 | 0 | 19 | 19 |
| Total | 194 | 39 | 19 | 252 |
| Group 3 – Control groupKappa coefficient for inter-rater reliability 1,000P <.0001 | ||||
| N0 | 352 | 0 | 0 | 352 |
| N1a | 0 | 56 | 0 | 56 |
| N1b | 0 | 0 | 29 | 29 |
| Total | 352 | 56 | 29 | 437 |
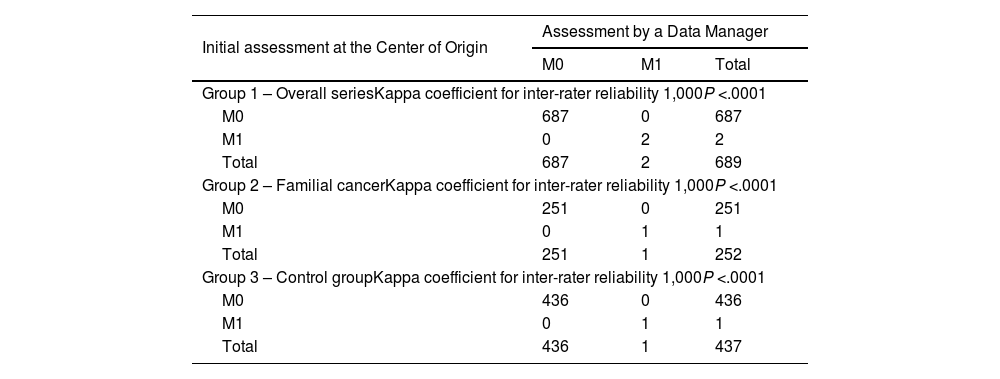
Data entries for the M component (systemic involvement) of the TNM system presented perfect agreement among the 3 groups (Tables 1 and 4).
Agreement between the initial assessment at the study center of origin and the centralized final assessment by a data manager regarding the M component M (systemic involvement) of the TNM system.
| Initial assessment at the Center of Origin | Assessment by a Data Manager | ||
|---|---|---|---|
| M0 | M1 | Total | |
| Group 1 – Overall seriesKappa coefficient for inter-rater reliability 1,000P <.0001 | |||
| M0 | 687 | 0 | 687 |
| M1 | 0 | 2 | 2 |
| Total | 687 | 2 | 689 |
| Group 2 – Familial cancerKappa coefficient for inter-rater reliability 1,000P <.0001 | |||
| M0 | 251 | 0 | 251 |
| M1 | 0 | 1 | 1 |
| Total | 251 | 1 | 252 |
| Group 3 – Control groupKappa coefficient for inter-rater reliability 1,000P <.0001 | |||
| M0 | 436 | 0 | 436 |
| M1 | 0 | 1 | 1 |
| Total | 436 | 1 | 437 |
Data entries for the TNM stage presented a disagreement rate of 2.5% (n = 17). This percentage was greater in the familial cancer group 2 (4%; n = 10) than in the sporadic carcinoma control group 3 (1.6%; n = 7), although said differences were not significant (P = .094) (Table 1).
Both globally (Kappa = 0.931) as well as in the familial cancer group 2 (Kappa = 0.904) and in control group 3 (Kappa = 0.950), agreement among the data entry managers was high (Table 5).
Agreement between the initial assessment at the study center of origin and the centralized final assessment by a data manager regarding staging according to the TNM system.
| Initial assessment at the Center of Origin | Assessment by a Data Manager | ||||
|---|---|---|---|---|---|
| I | II | III | IV | Total | |
| Group 1 – Overall seriesKappa coefficient for inter-rater reliability 0,931P <.0001 | |||||
| I | 540 | 4 | 3 | 0 | 547 |
| II | 1 | 44 | 1 | 0 | 46 |
| III | 3 | 0 | 59 | 5 | 67 |
| IV | 0 | 0 | 0 | 29 | 29 |
| Total | 544 | 48 | 63 | 34 | 689 |
| Group 2 – Familial cancerKappa coefficient for inter-rater reliability 0,904P <.0001 | |||||
| I | 186 | 2 | 1 | 0 | 189 |
| II | 1 | 24 | 0 | 0 | 25 |
| III | 2 | 0 | 24 | 4 | 30 |
| IV | 0 | 0 | 0 | 8 | 8 |
| Total | 189 | 26 | 25 | 12 | 252 |
| Group 3 – Control groupKappa coefficient for inter-rater reliability 0,950P <.0001 | |||||
| I | 354 | 2 | 2 | 0 | 358 |
| II | 0 | 20 | 1 | 0 | 21 |
| III | 1 | 0 | 35 | 1 | 37 |
| IV | 0 | 0 | 0 | 21 | 21 |
| Total | 355 | 22 | 38 | 22 | 437 |
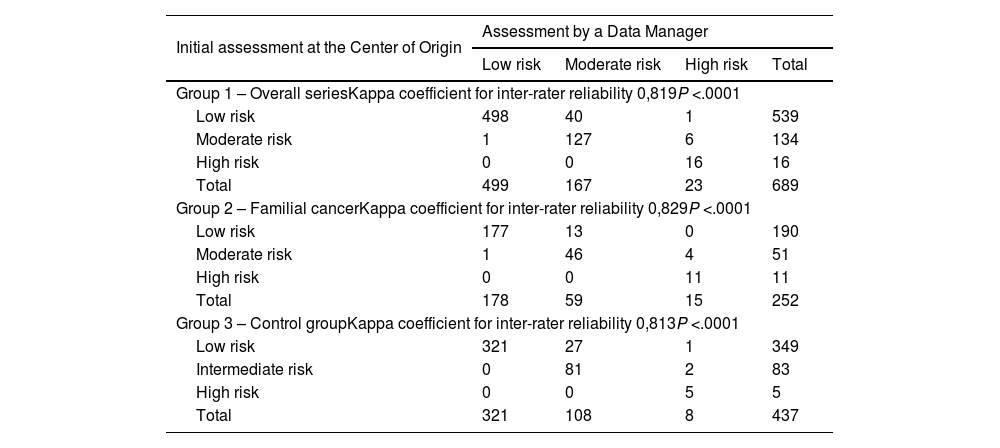
Data entries for the ATA risk of recurrence classification presented a disagreement rate of 7% (n = 48). In the familial cancer group 2, the discrepancies represented 7.1% (n = 18), while in the sporadic carcinoma control group 3, the discrepancies represented 6.9% (n = 30) of the cases (Table 1). These differences were not significant (P = .890).
Both at a global level (Kappa = 0.819) as well as in the familial carcinoma group 2 (Kappa = 0.829) and in control group 3 (Kappa = 0.813), agreement among the data entry operators was high (Table 6).
Agreement between the initial assessment at the study center of origin and the centralized final assessment by a data manager regarding the ATA risk of recurrence.
| Initial assessment at the Center of Origin | Assessment by a Data Manager | |||
|---|---|---|---|---|
| Low risk | Moderate risk | High risk | Total | |
| Group 1 – Overall seriesKappa coefficient for inter-rater reliability 0,819P <.0001 | ||||
| Low risk | 498 | 40 | 1 | 539 |
| Moderate risk | 1 | 127 | 6 | 134 |
| High risk | 0 | 0 | 16 | 16 |
| Total | 499 | 167 | 23 | 689 |
| Group 2 – Familial cancerKappa coefficient for inter-rater reliability 0,829P <.0001 | ||||
| Low risk | 177 | 13 | 0 | 190 |
| Moderate risk | 1 | 46 | 4 | 51 |
| High risk | 0 | 0 | 11 | 11 |
| Total | 178 | 59 | 15 | 252 |
| Group 3 – Control groupKappa coefficient for inter-rater reliability 0,813P <.0001 | ||||
| Low risk | 321 | 27 | 1 | 349 |
| Intermediate risk | 0 | 81 | 2 | 83 |
| High risk | 0 | 0 | 5 | 5 |
| Total | 321 | 108 | 8 | 437 |
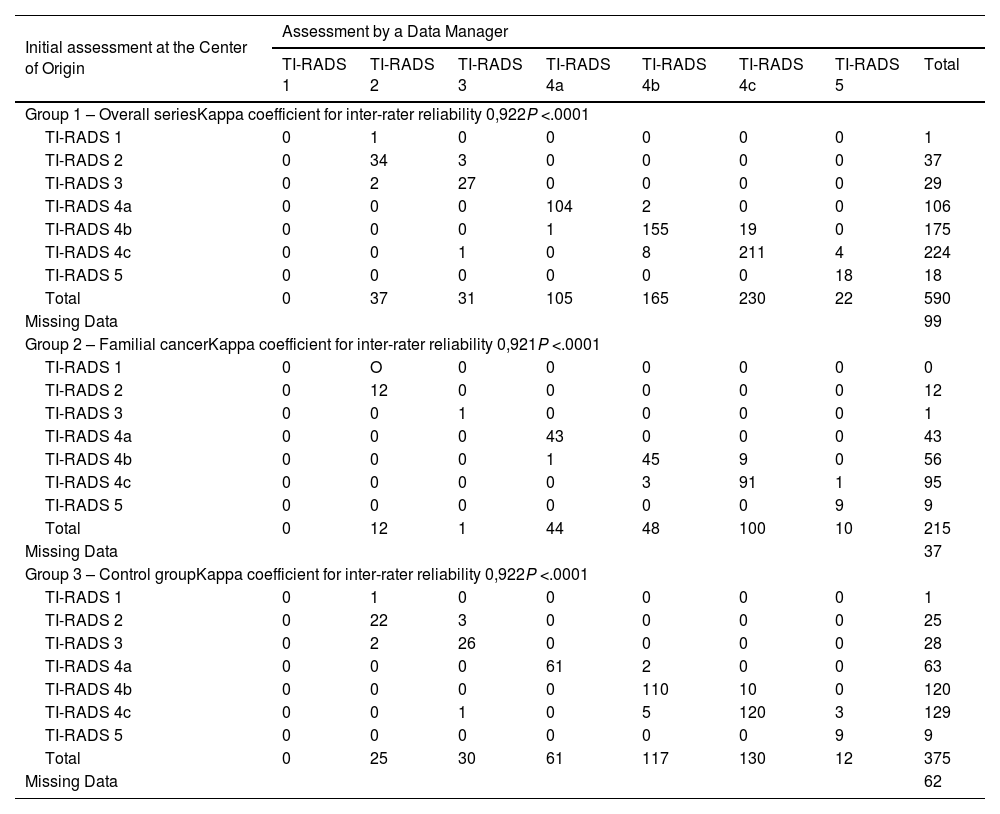
The assessment of the TI-RADS ultrasound classification presented a disagreement rate of 6.9% (n = 41). In the familial cancer group 2, the discrepancies were 6.5% (n = 14), while in the sporadic cancer control group 3, the discrepancies represented 7.2% (n = 27) of the cases (Table 1). These differences were not significant (P = .739).
Both overall (Kappa = 0.922) as well as in the familial cancer group 2 (Kappa = 0.921) and in control group 3 (Kappa = 0.922), agreement among the data processors was high (Table 7).
Agreement between the initial assessment at the study center of origin and the centralized final assessment by a data manager regarding the TI-RADS ultrasound score.
| Initial assessment at the Center of Origin | Assessment by a Data Manager | |||||||
|---|---|---|---|---|---|---|---|---|
| TI-RADS 1 | TI-RADS 2 | TI-RADS 3 | TI-RADS 4a | TI-RADS 4b | TI-RADS 4c | TI-RADS 5 | Total | |
| Group 1 – Overall seriesKappa coefficient for inter-rater reliability 0,922P <.0001 | ||||||||
| TI-RADS 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| TI-RADS 2 | 0 | 34 | 3 | 0 | 0 | 0 | 0 | 37 |
| TI-RADS 3 | 0 | 2 | 27 | 0 | 0 | 0 | 0 | 29 |
| TI-RADS 4a | 0 | 0 | 0 | 104 | 2 | 0 | 0 | 106 |
| TI-RADS 4b | 0 | 0 | 0 | 1 | 155 | 19 | 0 | 175 |
| TI-RADS 4c | 0 | 0 | 1 | 0 | 8 | 211 | 4 | 224 |
| TI-RADS 5 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 18 |
| Total | 0 | 37 | 31 | 105 | 165 | 230 | 22 | 590 |
| Missing Data | 99 | |||||||
| Group 2 – Familial cancerKappa coefficient for inter-rater reliability 0,921P <.0001 | ||||||||
| TI-RADS 1 | 0 | O | 0 | 0 | 0 | 0 | 0 | 0 |
| TI-RADS 2 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 12 |
| TI-RADS 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| TI-RADS 4a | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 43 |
| TI-RADS 4b | 0 | 0 | 0 | 1 | 45 | 9 | 0 | 56 |
| TI-RADS 4c | 0 | 0 | 0 | 0 | 3 | 91 | 1 | 95 |
| TI-RADS 5 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 9 |
| Total | 0 | 12 | 1 | 44 | 48 | 100 | 10 | 215 |
| Missing Data | 37 | |||||||
| Group 3 – Control groupKappa coefficient for inter-rater reliability 0,922P <.0001 | ||||||||
| TI-RADS 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| TI-RADS 2 | 0 | 22 | 3 | 0 | 0 | 0 | 0 | 25 |
| TI-RADS 3 | 0 | 2 | 26 | 0 | 0 | 0 | 0 | 28 |
| TI-RADS 4a | 0 | 0 | 0 | 61 | 2 | 0 | 0 | 63 |
| TI-RADS 4b | 0 | 0 | 0 | 0 | 110 | 10 | 0 | 120 |
| TI-RADS 4c | 0 | 0 | 1 | 0 | 5 | 120 | 3 | 129 |
| TI-RADS 5 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 9 |
| Total | 0 | 25 | 30 | 61 | 117 | 130 | 12 | 375 |
| Missing Data | 62 | |||||||
Transcription errors have been detected in 4.6% (n = 32) of cases, with similar percentages found in group 2 (4.8%; n = 12) and group 3 (4.6%; n = 20) (P = .939) (Table 1).
DiscussionIn clinical trials and multicenter studies, data collection notebooks or registers are basic tools used by researchers to record the clinical and non-clinical data of patients included in these studies. These data must then be registered in an electronic database for their analysis.10,11 The current trend is to record the data directly in the study database in order to facilitate the process.8 In addition, electronic data collection forms often contain verifications to reduce the entry of erroneous data, while offering administrators continuous information about the data and the collection process.4,12 However, their cost can be prohibitive in many multicenter clinical studies. Nevertheless, more and more computer platforms are being developed, so costs are expected to go down in the future.
Regardless of the type of data management, it is important to ensure that information is entered into the database in a consistent and accurate manner,13–15 as even a small rate of error can have a big impact on the results of a study.16 Random or systematic errors that go unnoticed are sources of bias in the database. Furthermore, the availability of reliable electronic systems is not enough to guarantee the validity of population-based cross-sectional studies. In fact, the relevance of a study largely depends on 2 main factors: quality data collection from the medical records, and the reliability of the data transferred from the medical records to the electronic system. Any weakness in these 2 stages will invalidate the study.16–19 Thus, the importance of training clinical researchers must be emphasized, particularly when it comes to training in good clinical practices.20–22
For all these reasons, although multicenter studies offer the advantage of obtaining information on rare pathologies in a relatively short period of time, they are more heterogeneous studies and have a greater risk of biases.1,3,22–24 Therefore, a balance must be sought between minimizing the potential biases of a study while not increasing the costs, which would make them unviable. In this context, the concept of centralized data entry by a data manager implies a more homogeneous protocol. However, it also entails a greater cost for the research project, which is sometimes unaffordable,1,3,22–24 and usually implies a longer data entry period versus when the protocol is followed independently by the different research groups.
Our multicenter project, which was conducted within the context of a national scientific society in Spain, shows how an adequate study design, detailed protocol, and complete explanation of the implications of each variable resulted in an acceptably high rate of agreement. Nevertheless, even though agreement was high (and in some cases perfect) for almost all the variables analyzed, the detailed analysis of the data revealed a percentage of disagreement that reached 7% for certain variables. Furthermore, these discrepancies were accentuated when the number of data processors was higher. Thus, we observed more disagreement in the familial cancer group 2, where there were 30 researchers entering data, versus the sporadic carcinoma control group 3, with only 4 researchers entering data.
In addition, the discrepancies mainly correlated with variables whose determination involves some type of nuance. For instance, agreement rates were almost perfect for concrete variables like lymph node involvement (N) or the presence of metastasis (M), with non-agreement rates of 0.7% and 0%, respectively. For the “T” variable, however, which is not only determined by size but also by factors such as the impact on neighboring structures, the non-agreement rate was greater than 4%. This situation became more obvious for variables where the classification was even more complex. Thus, we observed a disagreement rate of 7% for the ATA recurrence risk assessment and 6.9% in the TI-RADS ultrasound classification.
Therefore, although the agreement between direct data entry at the hospital of origin and centralized data processing by a data manager is high, there is a percentage of disagreement for important prognostic variables that ranges from 2.5% for TNM tumor stage to 7% for ATA the risk assessment. This implies a percentage of error or bias only in the process of data entry in multicenter studies. Thus, for these variables and although agreement is high, it is at the lower limit of said agreement — close to what is considered merely “good agreement”. To this, we must add the transcription error rate of 4.6%, as detected by the data manager.
Complying with data processing protocols is a slow and sometimes tedious process, and it is not always done by the main researcher at the hospital of origin, as the work is frequently delegated to others. All of this leads to possible errors in transcription. We should emphasize that the objective of this study is not to detect transcription errors, which suggests that this percentage may possibly be higher, since only evident data processing errors were detected. In order to detect all errors, the protocol variables would have to be reviewed in their entirety.
Hence, data collection must be well supervised to improve study quality. Although the use of a data manager makes no difference, the transcription error rate was nearly 5%. Therefore, it is necessary to create clear data entry protocols that are easy to complete, and we recommend that an experienced member of the data collection team review the main variables of the study. Otherwise, in the design phase of the study, the database should compile fewer variables but of greater quality and interest for the analysis.
We agree with other authors in that, if the staff members who log the data are well trained and supervised, this is not the weakest link in the data management chain,6,25,26 even though we have shown that this process does entail a margin of error.
One factor that our project has not corroborated (possibly because it is a short protocol) is that the position of the data entry field in the protocol may play an important role in the proportion of errors. The last few positions are associated with more errors than the initial fields, especially when the fields are numerical. This has been attributed to data manager fatigue when questionnaires have too many fields to complete.6 These results therefore results suggest that, to create more effective questionnaires, the most important information should be collected in the first few fields, the number of fields should be reduced, and “combo boxes” or text boxes should be used instead of fields with direct numerical insertion (especially in the last part of the questionnaire).
A limitation of this study is that it was based on a single data manager, making it difficult to generalize our conclusions. However, we should reiterate that the decision to use a single data manager was made to improve the reliability of data entry by reducing bias among data entry operators. To this point, it would be interesting to be able to compare our outcomes with other multicenter studies in other contexts for external validation of the results. Lastly, the quality of the data in multicenter studies with no data manager should be assessed, specifically regarding who collects the data (administrative staff, residents, etc) and under whose supervision, since these factors can influence the results, in which case the presence of a data manager could present differences.
Based on the data from our study, the existence of a data manager is not necessary given the high agreement between the data entry types, which represents savings in both time and costs.8 The drawbacks that online data processing may present can be resolved (as in our study) with standard databases that do not require direct costs or maintaining an online system, although it does not present the advantages offered by online products in terms of quality control. Nonetheless, they may be sufficient for multicenter projects like the one evaluated in our study. All these factors are important in multicenter studies of rare pathologies, where the contribution of cases is important, but at the same time funding is limited or even non-existent. In a Phase II Clinical Trial, data management costs can be almost 30% of the costs of Phase III clinical trials,8 and this added expense will subsequently impact the costs of marketed drugs. In multicenter clinical studies, these costs will determine the viability of the project itself.27
Lastly, it should be noted that, although there are no differences, and a data manager may not be necessary to log the data, it should be mentioned that data managers usually carry out other functions over the course of a project, such as coordinating patient testing, resolving problems that may arise in the participating study centers, serving as a liaison among researchers, etc. Therefore, if the budget of a project is able to cover the cost, hiring a data manager usually speeds up the completion of a project.
In conclusion, centralized data processing by a data manager in multicenter clinical studies seems to present similar results compared to direct data entry in the database at the hospital of origin.
Ethics approvalThe study protocol was approved by institute's committee.
Ethics Committee Code: 2021-2-13-HCUVA.
This human study has been reviewed by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 2000 Declaration of Helsinki as well as the Declaration of Istanbul 2008.
FundingNo specific funding sources have been used for this study.
PrécisIn multicenter clinical studies, the existence of a data manager is not required and the protocolization can be performed directly in the database in the center of origin due to the near perfect concordance between the two protocolization systems.
Authorship: Contribution to study by the Authors- 1
Conception and design: Ríos A.
- 2
Acquisition of a substantial portion of data: Rios A, Ferrero-Herrero E, Puñal-Rodríguez JA, Duran M, Mercader-Cidoncha E, Ruiz-Pardo J, Rodríguez JM.
- 3
Analysis and interpretation of data: Rios A, Gutiérrez PR.
- 4
Drafting of the manuscript: Rios A.
- 5
Critical revision of the manuscript for important intellectual content: Rios A, Gutiérrez PR, Mercader-Cidoncha E.
- 6
Statistical expertise: Rios A.
- 7
Obtaining funding for this project or study: Ríos A.
- 8
Supervision: Rios A, Ferrero-Herrero E, Puñal-Rodríguez JA, Gutiérrez PR, Durán M. Mercader-Cidoncha E, Ruiz-Pardo J, Rodríguez JM.
- 9
Final approval of the version to be published: Rios A, Ferrero-Herrero E, Puñal-Rodríguez JA, Gutiérrez PR, Mercader-Cidoncha E, Ruiz-Pardo J, Rodríguez JM.
The authors declare that they have no conflict of interest.
Thank the Spanish Association of Surgeons and the Endocrine Surgery Surgery Section for their support in carrying out this work. Thank the 24 Spanish Endocrine Surgery Units for having collaborated in the realization of this project and for having provided the data.