Assess the postoperative morbidity rates in pancreatic resection.
Material and methodProspective observational study which includes 117 patients who underwent surgery consecutively due to pancreatic or periampullary tumours. In 61 of the patients, cephalic pancreatectomy was carried out; 15 underwent total pancreatectomy; one underwent enucleation and 40 underwent distal pancreatectomy.
ResultsOverall morbidity was 48.7% (59% for cephalic pancreatectomy, 35% for distal pancreatectomy and 46.7% for total pancreatectomy). The most frequent complications were intra-abdominal abscesses and collections (15.38%) and medical complications (13.68%). The incidence of pancreatic fistula was 9.83% for cephalic pancreatectomy and 10% for distal pancreatectomy. The reintervention incidence was 14.53%. Overall mortality was 5.12% (6.56% for cephalic pancreatectomy, 2.5% for distal pancreatectomy and 6.67% for total pancreatectomy). The presence of postoperative complications, the need for reintervention and the fact of being over 70 years of age correlated significantly with mortality.
DiscussionPancreatic resection has high morbidity rates. Mortality is low and is practically limited to patients older than 70 years.
Evaluar la morbimortalidad postoperatoria de la resección pancreática.
Material y métodosEstudio observacional prospectivo que incluye 117 pacientes intervenidos quirúrgicamente de forma consecutiva por presentar tumoración pancreática o periampular. En 61 de ellos se hizo pancreatectomía cefálica; en 15, pancreatectomía total; en uno, enucleación y en 40, resección corporocaudal.
ResultadosLa morbilidad global fue de 48,7% (59% para la pancreatectomía cefálica, 35% para la resección corporocaudal y 46,7% para la pancreatectomía total). Las complicaciones más frecuentes fueron las colecciones y abscesos intraabdominales, con un 15,38% y las complicaciones médicas, con un 13,68%. La incidencia de fístula pancreática fue de 9,83%, para la pancreatectomía cefálica y de 10% para la resección corporocaudal. La incidencia de reintervención fue de 14,53%. La mortalidad global fue de 5,12% (6,56% para la pancreatectomía cefálica, 2,5% para la resección corporocaudal y 6,67% para la pancreatectomía total). La presencia de complicaciones postoperatorias, la necesidad de reintervención y la edad superior a 70 años correlacionaron significativamente con la mortalidad.
DiscusiónLa resección pancreática tiene una morbilidad alta. La mortalidad es baja y está prácticamente limitada a los pacientes mayores de 70 años.
Pancreatic resection is a technically complex operation, but its mortality rate has declined gradually to less than 5% reference centres.1–4 However, this procedure has a high incidence of postoperative complications that require the participation of an integrated and coordinated team of surgeons, radiologists, endoscopists and anaesthesiologists for proper management. Continuous improvements in the results obtained with this surgery in recent years are attributed to the incorporation of novel surgical techniques and perioperative management based on published experience. Therefore, access to the results in different centres will allow for comparisons that contribute to better outcomes. Few series have been published in the Spanish literature. Therefore, this study reports our postoperative complications and mortality results with pancreatic resection in our centre.
Patients and MethodsA prospective observational study was conducted. The study included 117 patients treated with pancreatic resection for pancreatic or periampullary tumours from January 2005 through December 2011. Data were collected prospectively from an anonymous database designed at the beginning of the study and analysed in June 2012. Demographic data, preoperative biliary drainage, surgical technique, transfusion requirements, complications, mortality and postoperative hospital stay were analysed.
Surgical TechniqueCephalic PancreatoduodenectomyA subcostal laparotomy is performed for cephalic pancreatoduodenectomy (CPD). The pancreaticoduodenal block is exposed to mobilise the hepatic flexure of the colon and to perform the Kocher manoeuvre to the left side of the aorta after metastatic disease is excluded. The interaortocaval lymph nodes, which are only analysed intraoperatively if they are >1cm in size, are resected at this time in continuity with the specimen. The omental cavity is accessed after sectioning the gastrocolic ligament, and the gastrocolic trunk is transected at the level of the superior mesenteric vein, which dissects the superior mesenteric vein and artery and transects the accessible branches. Antegrade cholecystectomy is performed, and the hepatic pedicle is dissected with skeletonisation of the vessels. The gastroduodenal artery is transected following confirmation of no obstruction in the celiac trunk using clamping. The bile duct is sectioned below the main biliary convergence, and its proximal portion remains occluded with a bulldog clamp until reconstruction. Hepatic pedicle lymphadenectomy extends from the biliary convergence to the origin of the hepatic artery. The gastric antrum is then resected and removed en bloc with the pancreatic head. Pyloric preservation is not performed. The pancreas is sectioned to the left of the portal vein, and pulsatile bleeding on the sectioned edge of the pancreatic body is monitored. An intraoperative biopsy of the pancreatic and biliary transection edges is performed in cases of malignant tumour. The jejunum is transected approximately 15cm from the ligament of Treitz and transposed to the right side of the mesenteric axis. Finally, the retroportal pancreatic lamina is sectioned to the right side of the superior mesenteric artery to complete the resection. Reconstruction starts with pancreatic anastomosis. Pancreatic duct-jejunal anastomosis was performed in all cases 3 using 6/0 polyglycolic acid interrupted stitches guided by an intraluminal drain tube approximately 6–8cm in length and size adapted to the diameter of the pancreatic duct. A catheter guide was not used in patients with a large diameter duct. The pancreatic remnant was invaginated into the stomach (1 case) or jejunum (2 cases) in patients with a duct diameter <1.5mm. End to side hepaticojejunostomy was performed at 8–15cm of the pancreatic anastomosis using 5/0 or 6/0 polyglycolic acid sutures. Finally, end to side gastrojejunostomy was performed using 4/0 running absorbable sutures. Child-type reconstruction was performed at the beginning of the series, but Roux-en-Y reconstruction with one loop for pancreatic and biliary trans-mesocolic anastomosis and another for gastrojejunal anastomosis in the antecolic position was performed in the last 2 years. Two closed drains, one peripancreatic and one subhepatic, were left in all cases.
Corporocaudal PancreatectomyAn attempt was made to preserve the spleen and the splenic vessels if the tumour was benign. The Warshaw technique was used if the splenic vessels could not be preserved. En-bloc splenectomy was performed in cases of malignant tumour. The operation begins with the sectioning of the splenic artery at its origin, sectioning of the neck of the pancreas and mobilisation of the surgical specimen from right to left. The pancreas is transected using an endostapler and reinforced with 3/0 single absorbable sutures if the resection is performed laparoscopically. A suction drain is left in the vicinity of the pancreatic remnant in all cases.
Total PancreatoduodenectomyThe procedure for total pancreatoduodenectomy is the same as that for corporocaudal pancreatectomy, and the adjacent spleen is removed. Total pancreatoduodenectomy was needed after CPD completion when the tumour affected the pancreatic edge. The procedure for total pancreatoduodenectomy begins as a CPD when this indication is considered from the onset, and the lack of vascular infiltration to contraindicate resection is confirmed. The splenic artery is severed at its origin, and the adjacent spleen and pancreas are mobilised from left to right. Finally, the retroportal lamina is sectioned. Reconstruction is performed identically to CPD, and one or two drains are left in place.
Multivisceral ResectionMultivisceral resection included the pancreas and the resection of another organ different from the gallbladder, duodenum, distal stomach or proximal jejunum.
Vascular ResectionVascular resection was indicated in cases with venous invasion without portal vein obstruction for patients ≤70 years of age. The procedure was contraindicated in cases with portal vein obstruction and invasion of the superior mesenteric artery, celiac trunk or hepatic artery. Invasion of the celiac trunk or hepatic artery proximal to the origin of the gastroduodenal artery was not a contraindication for corporocaudal pancreatectomy, provided that hepatic arterial flow through the gastroduodenal artery was detected. The procedure was contraindicated in patients older than 70 years in cases of CPD or total pancreatectomy.
Preoperative PreparationAntibiotic prophylaxis was used in all patients, and antibiotics were administered 1h before surgery. A combination of 2g of amoxicillin and 200mg of clavulanic acid or 1500mg of metronidazole and 2g of ceftriaxone was used. Prophylaxis was maintained for 24h. One dose of 40mg of enoxaparin and 100mcg of octreotide was also administered subcutaneously 12h before surgery.
Postoperative CareA nasogastric tube was not used routinely. Parenteral nutrition was used in patients with CPD and total pancreatectomy, which was maintained until the patient tolerated a semi-soft diet. The urinary catheter and central venous catheter were removed 24–48h after surgery, and an oral diet was initiated after 3–5 days. An oral diet was initiated 24h after surgery in the case of corporocaudal pancreatectomy. Octreotide (100mcg subcutaneously every 8h) was maintained for 7 days unless total pancreatectomy was performed. The first postoperative dose was administered at the end of surgery, and it was doubled in patients with soft pancreas and ducts less than 3mm. Heparin prophylaxis was maintained during hospitalisation in cases of benign tumours and for 30 days in cases of malignant tumours. The drains were removed between the third and eighth days, except in cases of biliary or pancreatic fistulas. Amylase and bilirubin in drain contents and any drain collections were determined from the third day onwards. Prokinetic agents were not used routinely.
Morbidity and MortalityPancreatic fistula was defined as amylase levels in the contents of the drain or any drain collection >3 times the serum values after the third day.5
Biliary fistula was defined as bilirubin levels in the contents of the drain or drain collection >3 times the serum values after the third day.6
Delayed gastric emptying was diagnosed when continued nasogastric tube was required from the third day onwards or the patient was intolerant to oral feeding beyond the seventh postoperative day.7
Postoperative bleeding was considered if there was a decrease of Hb levels >3g/dL.8
Statistical AnalysisStatistical analysis of the data was performed using IBM SPSS (Statistical Package for Social Sciences Inc., Chicago, Illinois, USA), Version 20.0 for Mac. A descriptive analysis of the variables included in the study was performed. The Shapiro–Wilk and Kolmogorov tests determined whether quantitative variables conformed to a normal distribution. For continuous quantitative variables, measures of central tendency and dispersion were obtained, expressed as means with 95% confidence intervals (CIs) in the case of normally distributed variables or as medians. For qualitative variables, proportions with 95% CIs were determined. Fisher's exact test compared qualitative variables in the case of non-normal distribution. The Kruskal–Wallis test or Mann–Whitney test compared quantitative variables between groups of patients, and the Spearman rank correlation coefficient compared 2 quantitative variables. The chi-square test compared qualitative variables in the case of normal distribution of adjusted variables. The Student's t-test compared quantitative variables, and the Pearson correlation coefficient compared 2 quantitative variables. A logistic regression model in which the variables of the P value were less than .20 analysed the variables related to mortality. Statistical significance was defined as P value <.05.
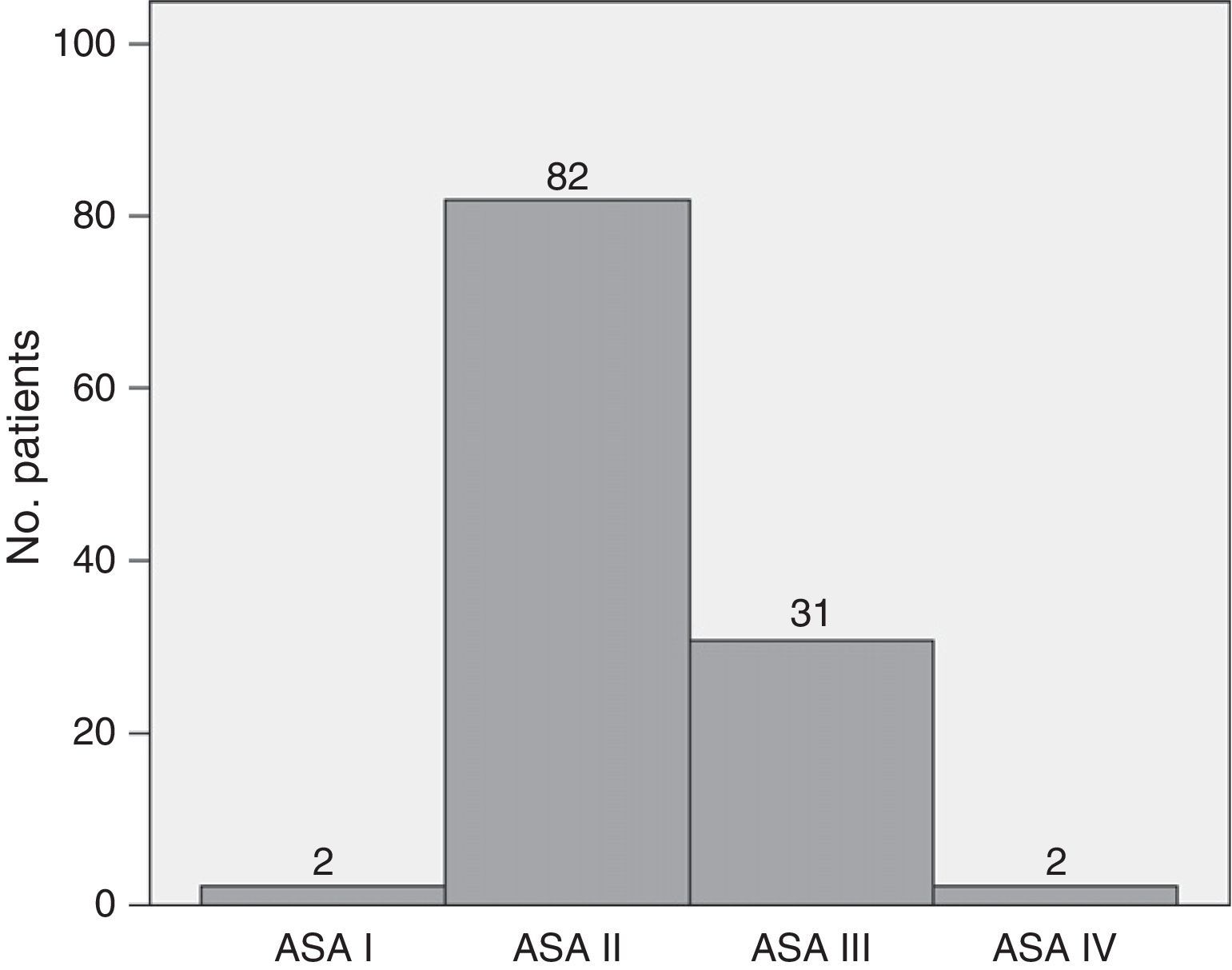
ResultsThe series included 117 patients with a median age of 64.38 years (95% CI: 62.3–66.47). The youngest patient was 34 years old, and the oldest patient was 82. Of the patients, 53.8% were male, and 46.2% were female. Mean preoperative BMI was 25.97 (95% CI 25.36–26.59). Fig. 1 shows that 70.1% of patients were ASA II, and 26.5% were ASA III. The Charlson index had a median of 0 with a 75th percentile of 1. Approximately 71% of patients had a Charlson score of 0, 17.9% had a Charlson score of 1, 10.25% had a score of 2, and only one patient had a score of 3. Preoperative biliary drainage was performed in 31 patients (26.5%), via endoscopy in 29 patients and percutaneously in 2 patients.
CPD was the surgical technique in 61 patients (52.1%), and corporocaudal pancreatectomy was used in 40 patients (34.2%). Total pancreatectomy was used in 15 (12.8%), and enucleation was performed in one patient (0.9%). Laparoscopic corporocaudal pancreatectomy was performed in 15 patients (37.5%), and the spleen was preserved in 6 of these patients (40%). The splenic vessels were preserved in 4 of these patients, and the Warshaw technique was used in 2 patients. Resection of the portal vein or superior mesenteric vein was performed in 4 patients (2 patients with CPD and 2 with total pancreatectomy). Thirteen (11.1%) patients underwent multivisceral resection (6 with CPD, 4 with corporocaudal pancreatectomy, and 3 with total pancreatectomy). Forty patients (34.18%) required an intraoperative transfusion with a median of one unit of packed red blood cells (1–7). Throughout the postoperative period, 42 (35.9%) patients were transfused with a median of 2 units of packed red blood cells (1–27). Sixty-three patients had a transfusion during the perioperative period (53.8%). Total pancreatectomy patients most frequently required transfusion during surgery and postoperatively (60% and 46.7%, respectively), but these patients only significantly required transfusion in the intraoperative period (P=.03). Corporocaudal pancreatectomy patients less frequently required transfusion (29% and 30%, respectively), but these values were not significant. CPD patients required intraoperative transfusion in 31.14% of cases and postoperative transfusion in 37.7% of cases. Intraoperative transfusion was significantly associated with the type of resection (P=.013), venous resection (P=.017) and multivisceral resection (P=.011). Intraoperative transfusion in corporocaudal pancreatectomy was significantly lower when the laparoscopic approach was used.
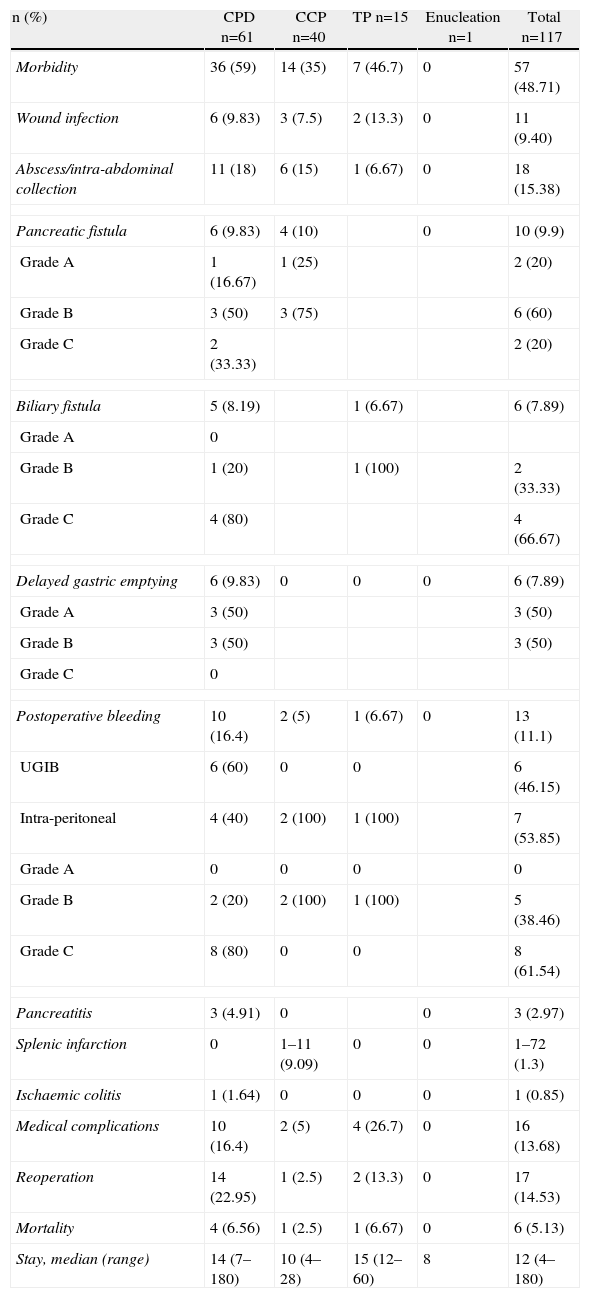
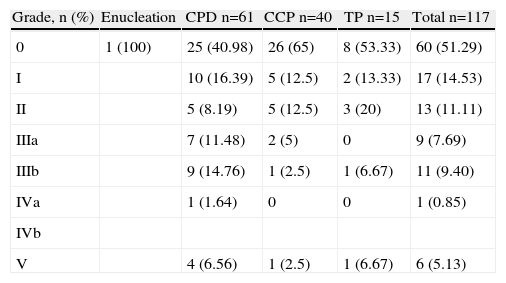
The overall incidence of complications was 48.7%. The most frequent complications were intra-abdominal abscesses and collections (15.38%) and medical complications (13.68%). Table 1 shows morbidity and mortality data according to the type of resection. Table 2 shows morbidity according to the Clavien–Dindo classification,9 which accounts for its importance and the treatment required for control and resolution. None of the variables were significantly associated with overall morbidity. Intra-abdominal abscess and biliary fistula were significantly more common when multivisceral resection was performed (P=.029 and P=.018, respectively). Pancreatic fistula was not significantly associated with any of the studied variables.
Morbidity and Mortality of Pancreatic Resection.
| n (%) | CPD n=61 | CCP n=40 | TP n=15 | Enucleation n=1 | Total n=117 |
| Morbidity | 36 (59) | 14 (35) | 7 (46.7) | 0 | 57 (48.71) |
| Wound infection | 6 (9.83) | 3 (7.5) | 2 (13.3) | 0 | 11 (9.40) |
| Abscess/intra-abdominal collection | 11 (18) | 6 (15) | 1 (6.67) | 0 | 18 (15.38) |
| Pancreatic fistula | 6 (9.83) | 4 (10) | 0 | 10 (9.9) | |
| Grade A | 1 (16.67) | 1 (25) | 2 (20) | ||
| Grade B | 3 (50) | 3 (75) | 6 (60) | ||
| Grade C | 2 (33.33) | 2 (20) | |||
| Biliary fistula | 5 (8.19) | 1 (6.67) | 6 (7.89) | ||
| Grade A | 0 | ||||
| Grade B | 1 (20) | 1 (100) | 2 (33.33) | ||
| Grade C | 4 (80) | 4 (66.67) | |||
| Delayed gastric emptying | 6 (9.83) | 0 | 0 | 0 | 6 (7.89) |
| Grade A | 3 (50) | 3 (50) | |||
| Grade B | 3 (50) | 3 (50) | |||
| Grade C | 0 | ||||
| Postoperative bleeding | 10 (16.4) | 2 (5) | 1 (6.67) | 0 | 13 (11.1) |
| UGIB | 6 (60) | 0 | 0 | 6 (46.15) | |
| Intra-peritoneal | 4 (40) | 2 (100) | 1 (100) | 7 (53.85) | |
| Grade A | 0 | 0 | 0 | 0 | |
| Grade B | 2 (20) | 2 (100) | 1 (100) | 5 (38.46) | |
| Grade C | 8 (80) | 0 | 0 | 8 (61.54) | |
| Pancreatitis | 3 (4.91) | 0 | 0 | 3 (2.97) | |
| Splenic infarction | 0 | 1–11 (9.09) | 0 | 0 | 1–72 (1.3) |
| Ischaemic colitis | 1 (1.64) | 0 | 0 | 0 | 1 (0.85) |
| Medical complications | 10 (16.4) | 2 (5) | 4 (26.7) | 0 | 16 (13.68) |
| Reoperation | 14 (22.95) | 1 (2.5) | 2 (13.3) | 0 | 17 (14.53) |
| Mortality | 4 (6.56) | 1 (2.5) | 1 (6.67) | 0 | 6 (5.13) |
| Stay, median (range) | 14 (7–180) | 10 (4–28) | 15 (12–60) | 8 | 12 (4–180) |
CPD, cephalic pancreatoduodenectomy; UGIB, upper gastrointestinal bleeding, CCP, corporocaudal pancreatectomy, TP, total pancreatectomy.
Morbidity According to the Clavien Classification.
| Grade, n (%) | Enucleation | CPD n=61 | CCP n=40 | TP n=15 | Total n=117 |
| 0 | 1 (100) | 25 (40.98) | 26 (65) | 8 (53.33) | 60 (51.29) |
| I | 10 (16.39) | 5 (12.5) | 2 (13.33) | 17 (14.53) | |
| II | 5 (8.19) | 5 (12.5) | 3 (20) | 13 (11.11) | |
| IIIa | 7 (11.48) | 2 (5) | 0 | 9 (7.69) | |
| IIIb | 9 (14.76) | 1 (2.5) | 1 (6.67) | 11 (9.40) | |
| IVa | 1 (1.64) | 0 | 0 | 1 (0.85) | |
| IVb | |||||
| V | 4 (6.56) | 1 (2.5) | 1 (6.67) | 6 (5.13) |
CPD, cephalic pancreatoduodenectomy; CCP, corporocaudal pancreatectomy, TP, total pancreatectomy.
Overall mortality was 5.12% (6 patients). Mortality was 6.56% for CPD (4 patients), 2.5% (one patient) for corporocaudal pancreatectomy and 6.67% (one patient) for total pancreatectomy. The presence of postoperative complications, need for reoperation and age older than 70 years significantly correlated with mortality (P=.005, P<.0001 and P=.007, respectively). Multivariate analysis revealed that only the need for reoperation remained significant (OR: 23.29, 95% CI: 2.26–239.68).
The reconstruction technique using the Child or Roux-en-Y techniques and the size of the tumour were not associated with postoperative complications or mortality.
Median overall hospital stay was 12 days (4–180). Median hospital stay was 14 (4–180) for CPD, 15 (9–67) for total pancreatectomy and 10 (4–42) for corporocaudal pancreatectomy (P=.002). Hospital stay was also significantly higher in patients with multivisceral resection (P=.037) considering overall complications (P=.000), intra-abdominal abscess (P<.0001), reoperation (P=.001), pancreatic fistula (P=.015) and the severity of complications (P<.0001).
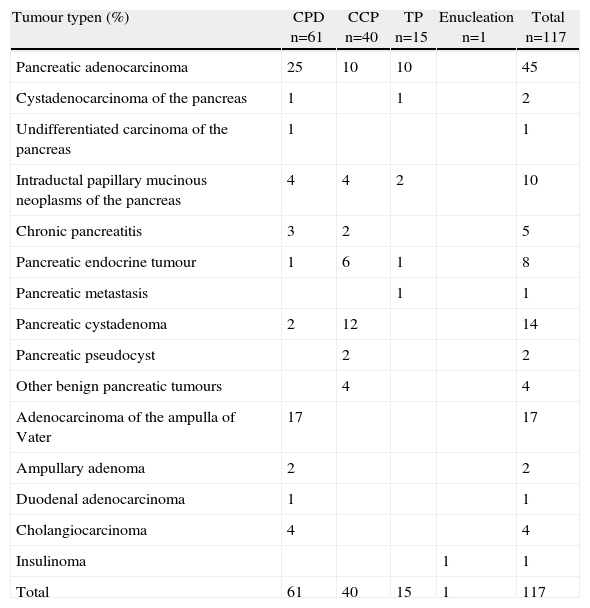
The most common indication for pancreatic resection was pancreatic adenocarcinoma (Table 3). Only 3 patients underwent neoadjuvant therapy: two patients had no postoperative complications, and the third patient presented an intra-abdominal abscess.
Histological Diagnosis.
| Tumour typen (%) | CPD n=61 | CCP n=40 | TP n=15 | Enucleation n=1 | Total n=117 |
| Pancreatic adenocarcinoma | 25 | 10 | 10 | 45 | |
| Cystadenocarcinoma of the pancreas | 1 | 1 | 2 | ||
| Undifferentiated carcinoma of the pancreas | 1 | 1 | |||
| Intraductal papillary mucinous neoplasms of the pancreas | 4 | 4 | 2 | 10 | |
| Chronic pancreatitis | 3 | 2 | 5 | ||
| Pancreatic endocrine tumour | 1 | 6 | 1 | 8 | |
| Pancreatic metastasis | 1 | 1 | |||
| Pancreatic cystadenoma | 2 | 12 | 14 | ||
| Pancreatic pseudocyst | 2 | 2 | |||
| Other benign pancreatic tumours | 4 | 4 | |||
| Adenocarcinoma of the ampulla of Vater | 17 | 17 | |||
| Ampullary adenoma | 2 | 2 | |||
| Duodenal adenocarcinoma | 1 | 1 | |||
| Cholangiocarcinoma | 4 | 4 | |||
| Insulinoma | 1 | 1 | |||
| Total | 61 | 40 | 15 | 1 | 117 |
The indications for pancreatic resection have expanded in recent decades, primarily for 3 reasons. First, pancreatic cancers are currently removed surgically, when resection was previously contraindicated. Second, tumours that were diagnosed less frequently, such as intraductal papillary mucinous neoplasms, undergo. Third, older patients, including 90-year-old patients, undergo surgery.10 The most common indication for surgery in our series was pancreatic adenocarcinoma, which is consistent with previous reports.11 However, a relatively large number of patients with intraductal papillary mucinous neoplasm and patients with neuroendocrine tumours were included in this study. This expansion in the indications for pancreatic resection is attributed to recent improvements in outcomes. The quality standard for CPD is mortality <10% and morbidity <50%,12 but centres with a high volume of patients exhibit mortality <5%1–3 and even around 1%.4 The overall mortality was 5.12% in our series. Mortality was 6.56% for CPD and 6.67% and 2.5% for total pancreatectomy and corporocaudal resection, respectively. These data are similar to recent reports of other Spanish authors.11–14 Mortality in our study was significantly higher in patients older than 70 years, patients with postoperative complications and patients requiring reoperation, which is consistent with previously published data.14,15 However, the only variable that remained significantly associated with mortality in multivariate analysis was reoperation. Pancreatic fistula is a leading cause of death,16 but we found no significant relationship in our series. The recent reduction in mortality is linked primarily to improvements in surgical technique, postoperative care and the management of complications. However, mortality reductions have not been accompanied by a substantial reduction in the incidence of complications, which is still very high. This incidence is 38% and 46%, respectively, in centres with extensive experience and excellent reputations, such as Johns Hopkins and Memorial Sloan-Kettering Center.17,18 The incidence of complications is especially high in CPD and total pancreatectomy. The overall complication rate in our series was 48.7%, with 59% for CPD, 46.7% for total pancreatectomy and 35% for corporocaudal resection. These data are consistent with previously published data.11,14 Pancreatic fistula, postoperative bleeding and intra-abdominal abscesses and collections were particularly relevant complications of pancreatic resection. The incidence of pancreatic fistula varies greatly between series and according to the type of study, and it tends to be higher in prospective randomised studies. Released figures typically range between 5 and 20% for CPD. The incidence for CPD was 9.83% in our series, which is similar to larger series.14,19,20 Higher numbers for distal pancreatectomy, between 20% and 40%, are usually published.20 The incidence of pancreatic fistula in our series was only 10% for corporocaudal pancreatectomy, which is similar to other authors.11 Previously published studies have found that males, BMI>30, soft pancreas and pancreatic duct diameter <3mm are risk factors for the occurrence of pancreatic fistula.21 However, no significant relationship with the occurrence of this complication was observed in our series, most likely due to the small number of cases. Pancreatic fistulas have a special significance in CPD because their consequences are more serious. Several types of pancreatic anastomosis have been designed to reduce the incidence of pancreatic fistula in these patients, but none has demonstrated superiority.22 The most important factor for surgical outcome is meticulous technique,11 and we used loops of 3.5× to create pancreatic and biliary anastomosis routinely. We performed pancreatic anastomosis using invagination into the stomach or jejunum when the pancreatic duct diameter was excessively thin. No differences in the incidence of pancreatic fistula in corporocaudal pancreatectomy according to the type of closure of the proximal pancreatic remnant have been reported.23 Intra-abdominal abscesses are particularly common in CPD, affecting up to 29% of patients.24 However, the incidence is generally lower, between 5% and 10%. The incidence in our series was 18% for CPD and 15% for corporocaudal resection, which is somewhat higher than usually published figures. The only variable that demonstrated a statistically significant association with the occurrence of intra-abdominal abscess in our study was multivisceral resection. Postoperative bleeding is one of the surgeon's most feared complications. This complication is rare in corporocaudal resection, but the incidence is usually between 5% and 12% in CPD and total pancreatectomy.25 Bleeding may be intraluminal, and it usually arises from a suture line or intra-abdominally. Bleeding may be early or late (after the fifth postoperative day). The latter is often associated with the presence of a pancreatic fistula.25 The incidence of postoperative bleeding in our series is similar to previous reports.
Other complications associated with CPD and total pancreatectomy are delayed gastric emptying and biliary fistula. The incidence of delayed gastric emptying in our series was approximately 10%, which is an average value compared with other authors.11,13,14 This finding could be at least partially explained because pyloric preservation was not performed in any patient. We routinely perform antrectomy in pancreatoduodenectomy because there is no evidence that pyloric preservation reduces morbidity and mortality or improves the quality of life and nutritional status of the patient.26 The incidence of biliary fistula in our series was somewhat higher than previous reports, but half of the cases occurred after reoperation was performed for another reason. Reducing morbidity is difficult, but some authors have observed that the non-use of drains significantly reduces this complication and decreases the incidence of fistulas and abscesses.13
The high incidence of serious surgical complications causes a higher reoperation rate, which was nearly 15% for all pancreatic resections and almost 23% for CPD in our series. This incidence of reoperation is higher than previous publications, which is approximately 10% for CPD.25 The reoperation rate is related not only to the incidence of serious complications, such as pancreatic fistula, postoperative bleeding and intra-abdominal abscesses, but also to the rate of resolution of these complications using nonsurgical procedures. Our centre is trying to enhance endoscopic drainage of peripancreatic collections and improve the success rate with percutaneous drainage to reduce the high rate of repeat interventions and reduce mortality to <5%.
The postoperative hospital stay of these patients is usually prolonged. The median hospital stay for all pancreatic resections in our series was 12 days and was 14 days for CPD, which was significantly higher in cases of multivisceral resection, intra-abdominal abscess, pancreatic fistula and overall morbidity according to the severity of complications and the need for reoperation. These findings are consistent with other authors.14 Recent fast track surgery programmes have been launched to reduce hospital stay and postoperative complications, as revealed in our country by the Navarra University Clinic Group.13
In conclusion, pancreatic resection, especially pancreatoduodenectomy, continues to have significant morbidity and mortality and prolonged hospital stays, but this should not lead to therapeutic nihilism, and resection is the only chance to obtain prolonged survival in cases of pancreatic or biliopancreatic junction malignancy.11 Pancreatoduodenectomy mortality is higher in patients older than 70 years, but this procedure is indicated in this population, except in patients with significant comorbidity.
Conflicts of InterestThe authors declare no conflicts of interest.
Please cite this article as: Dominguez-Comesaña E, Gonzalez-Rodriguez FJ, Ulla-Rocha JL, Lede-Fernandez Á, Portela-Serra JL, Piñon-Cimadevila MÁ. Morbimortalidad de la resección pancreática. Cir Esp. 2013;91:651–658.