Despite limited published evidence, robotic-assisted thoracoscopic surgery (RATS) for anatomic lung resection in early-stage lung cancer continues growing. The aim of this study is to evaluate its safety and oncologic efficacy compared to video-assisted thoracoscopic surgery (VATS).
MethodsSingle-centre retrospective study of all patients with resected clinical stage IA NSCLC who underwent RATS or VATS anatomic lung resection from June 2018 to January 2022. RATS and VATS cases were matched by propensity scoring (PSM) according to age, sex, histology, and type of resection. Short-term outcomes were compared, and the Kaplan-Meier method and log-rank test were used to evaluate the overall survival (OS) and disease-free survival (DFS).
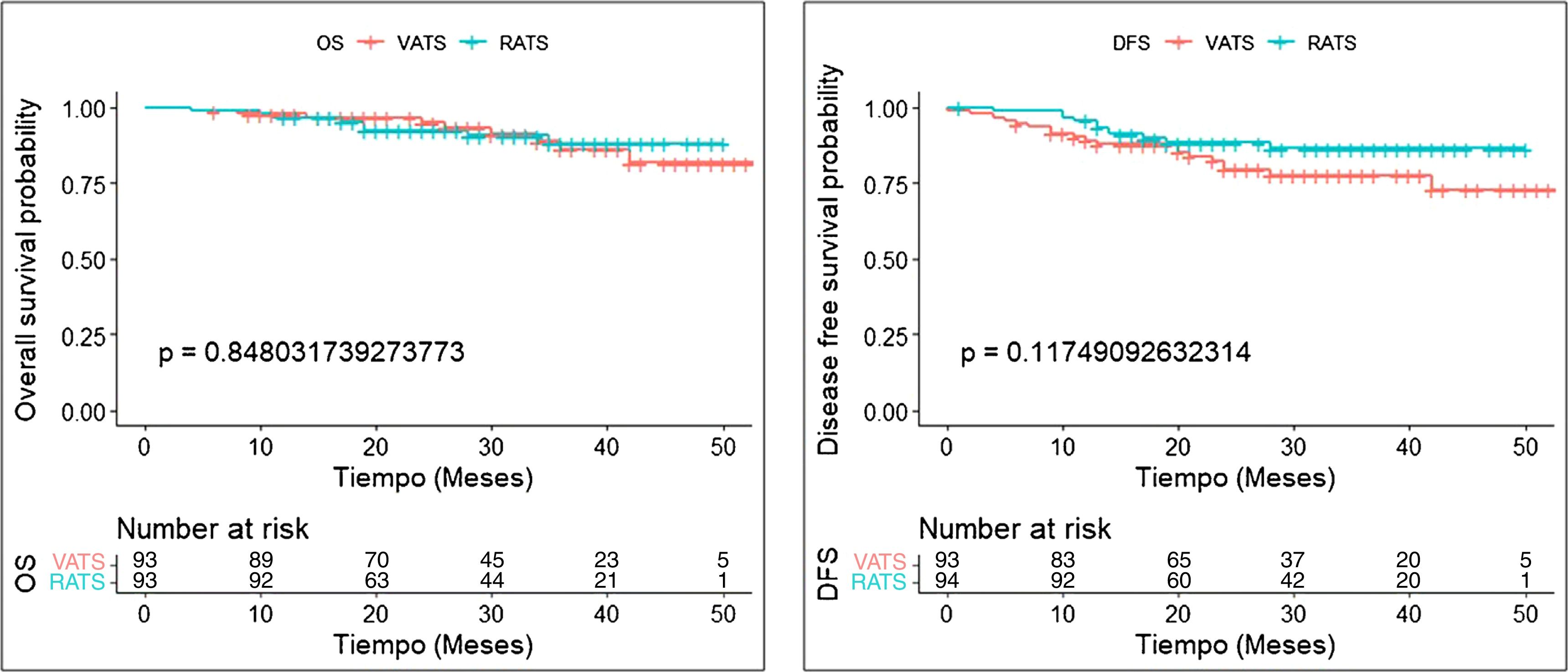
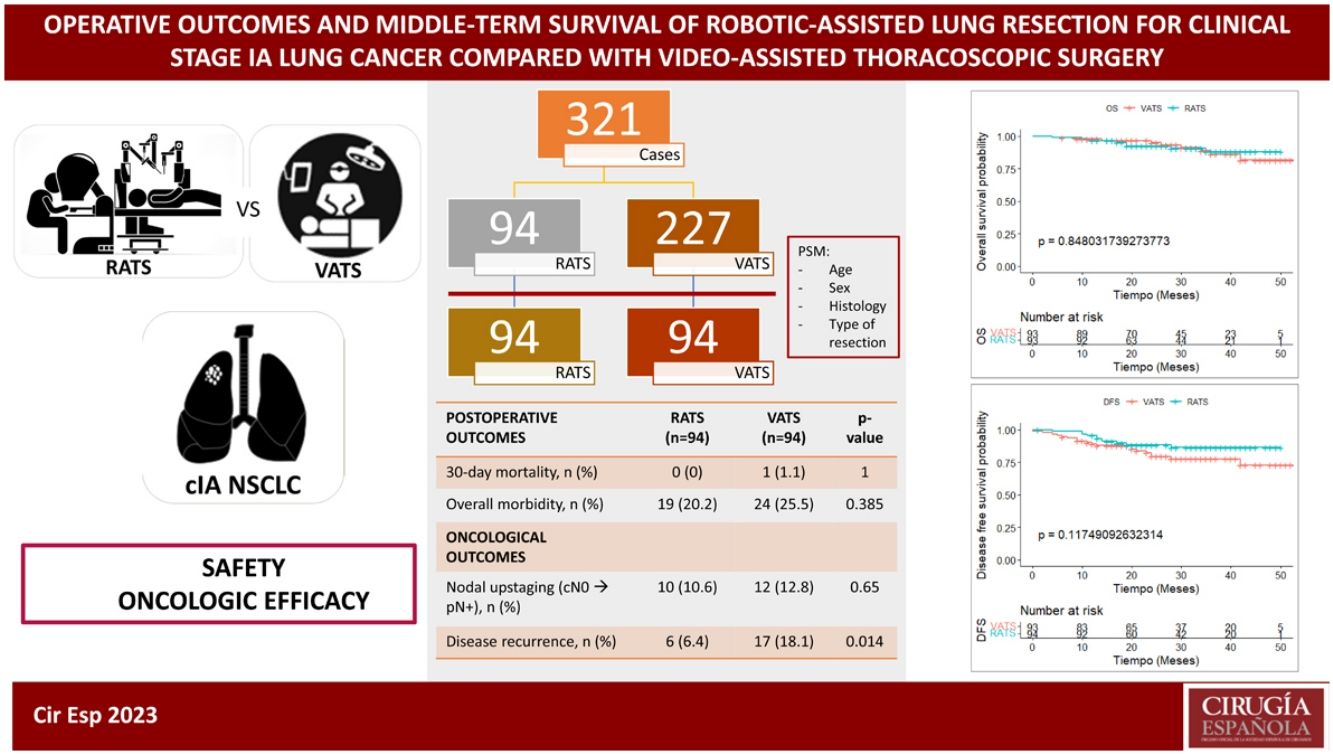
Results321 patients (94 RATS and 227 VATS cases) were included. After PSM, 94 VATS and 94 RATS cases were compared. Demographics, pulmonary function, and comorbidity were similar in both groups. Overall postoperative morbidity was comparable for RATS and VATS cases (20.2% vs 25.5%, P = 0.385, respectively). Pathological nodal upstaging was similar in both groups (10.6% in RATS and 12.8% in VATS). During the 3.5-year follow-up period (median: 29 months; IQR: 18–39), recurrence rate was 6.4% in RATS group and 18.1% in the VATS group (P = 0.014). OS and DFS were similar in RATS and VATS groups (log rank P = 0.848 and P = 0.117, respectively).
ConclusionRATS can be performed safely in patients with early-stage NSCLC. For clinical stage IA disease, robotic anatomic lung resection offers better oncologic outcomes in terms of recurrence, although there are no differences in OS and DFS compared with VATS.
A pesar de la limitada evidencia disponible, el uso de la RATS en resecciones pulmonares anatómicas por cáncer continúa creciendo. El objetivo de este estudio es evaluar su seguridad y eficacia oncológica en comparación con la VATS.
MétodosEstudio retrospectivo unicéntrico en el que se incluyeron todos los pacientes con CPNM en estadio cIA sometidos a resección pulmonar anatómica RATS o VATS entre junio de 2018 y enero de 2022. Los casos se emparejaron mediante puntuación de propensión (PSM) según edad, sexo, histología y tipo de resección. Se compararon los resultados a corto plazo y la supervivencia global (OS) y libre de enfermedad (DFS) mediante el método de Kaplan-Meier y la prueba de rangos logarítmicos.
ResultadosSe incluyeron 321 pacientes (94 RATS y 227 VATS). Tras el PSM, se compararon 94 VATS y 94 RATS. La morbilidad global fue comparable en ambos grupos (20.2 % en RATS vs 25.5 % en VATS, P = 0.385). El upstaging ganglionar fue similar en ambos abordajes (10.6% en RATS y 12.8% en VATS). Durante los 3.5 años de seguimiento, la tasa de recurrencia fue del 6.4 % en RATS y del 18.1 % en VATS (P = 0.014). OS y DFS fueron similares en los dos grupos (rango logarítmico P = 0.848 y P = 0.117, respectivamente).
ConclusiónLa RATS se puede realizar de forma segura en pacientes con CPNM en estadio inicial. Para la enfermedad en estadio cIA, el abordaje robótico ofrece mejores resultados en términos de recurrencia, aunque no hay diferencias en la OS y la DFS en comparación con la VATS.
According to last reported data, lung cancer continues being the leading cause of cancer-related death worldwide1 with a 5-year net survival rate lower than 24%.2 This poor survival rate is mainly due to the fact that in most countries less than 20% of lung cancer patients are diagnosed at stage I when the disease is still potentially resectable and curable.3 However, in the last decade, the development of lung cancer screening programs whose aim is reducing mortality in high-risk individuals through early detection, has led to an increased identification of small nodules and consequently to an enhanced diagnosis of early-stage lung cancer.4
On the other hand, surgical removal of the tumour remains the standard of care in patients with early-stage non-small cell lung cancer (NSCLC)5 and minimally invasive techniques such as video-assisted thoracoscopic surgery (VATS) have become the gold standard approach for its surgical treatment.5,6 Additionally, robot-assisted thoracic surgery (RATS) has recently emerged as an alternative minimally invasive approach for the treatment of patients with pulmonary malignancies and although robotic-assisted systems provide surgeons with some technical advantages over VATS approach,7,8 comparative studies between VATS and RATS are still limited in number and provide mixed and conflicting results. Regarding short-term outcomes, the superiority of VATS or RATS remains unclear after the publication of two randomized controlled trials9,10 and several systematic reviews and meta-analysis11–17 mainly based on retrospective series and propensity matched cohorts. Also, some studies have focused on comparing long-term oncological outcomes of patients undergoing anatomical lung resection for early-stage NSCLC based on the surgical minimally invasive approach (VATS or RATS).18–23
Given the controversial results reported in previous studies, we intend to evaluate our institutional outcomes. Therefore, in this study we compare the perioperative outcomes, long-term overall survival (OS) and disease-free survival (DFS) of patients diagnosed with clinical stage IA NSCLC undergoing robotic anatomical lung resection with those of a propensity score matched group of patients treated with VATS with the purpose of comparing safety and oncologic effectiveness of RATS to VATS.
Our manuscript is reported according to the STROBE recommendations.
MethodsStudy design, data source and patientsA single-centre observational, retrospective, and comparative cohort study was conducted. All data were obtained from an institutional prospective, computerized database. Quality control of the data was assured by two successive audits made by the quality control manager of the unit, the first one at the moment of patient hospital discharge and the second before uploading the final records in the hospital central archive. The database included clinical and pathological variables, type of resection, intraoperative and postoperative data, complications, recurrence, and survival. Definition of variables were based on the standardized document of the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons (ESTS)24 and the classification of surgical complications proposed by Dindo et al.25
The inclusion criteria consisted of patients aged ≥18 years old, who underwent VATS or RATS anatomical lung resection (segmentectomy, lobectomy or bilobectomy) with curative intent for a cito-histologically diagnosed clinical stage IA NSCLC (equal to or less than 3 cm in size, N0 and no signs of pleural involvement) between June 2018 and December 2022 in our centre. Patients who underwent pneumonectomy or received induction treatment were excluded from the study.
The preoperative staging was performed by routine computed tomography (CT) scan, brain magnetic resonance imaging or a cerebral CT scan and a fluorodeoxyglucose positron emission tomography scan (PET scan). For patients with abnormal mediastinal and/or hilar lymph nodes (LN) at CT and/or PET, endobronchial ultrasound (EBUS) was indicated for mediastinal and hilar staging. In case of negative EBUS and a highly suspicious malignant LN, mediastinoscopy was performed. Preoperative and postoperative staging was done using the 8th edition of the tumour, nodes, and metastasis (TNM) classification.26
Operative proceduresThe choice of minimally invasive surgical approach (VATS or RATS) was principally based on robot equipment availability, but not on tumour or patients’ characteristics. All VATS procedures were performed by a team of five board certified thoracic surgeons. RATS anatomical resections were performed by two members of the same team who were Intuitive Surgical certified surgeons. Operative technique was standardized and homogeneous for both approaches as it was previously described.27
Postoperative managementPerioperative management was uniform for all patients throughout the study period. Antibiotic prophylaxis consisted in one single dose of cefazoline 2 g, repeated after 6 h if surgery continued. Systematic nodal dissection was performed according to the guidelines of the ESTS.28 Patients were extubated in the operating room and, after 6 h in the recovery room, transferred to the thoracic ward. At the beginning of the procedure, under direct vision, a paravertebral catheter was inserted for postoperative analgesia with bupivacaine and fentanyl infusion for a maximum of three days postoperatively. Oral paracetamol and non-steroid anti-inflammatory drugs were indicated thereafter. Nursing care was homogeneous in all cases and including incentive spirometry, early mobilisation and standardised intensive physiotherapy was indicated in all patients.29
Follow-upShort-term follow-up of thoracic surgeons was prospectively recorded in our institutional database. Middle-term follow-up data was retrospectively completed from 2018 to January 2023 by reviewing the patients’ electronic records.
OutcomesThe primary endpoint was operative overall morbidity, defined as any adverse event occurred within 30 days after the operation, or later if the patient was still in the hospital and included pulmonary, cardiovascular, metabolic, gastrointestinal, urological, hematologic, neurological, and psychiatric complications and wound infection. Secondary endpoints were cardiopulmonary and major complications. Cardiopulmonary complications included respiratory failure, need for reintubation, prolonged mechanical ventilation >24 h, pneumonia, atelectasis requiring bronchoscopy, pulmonary oedema, pulmonary embolism, ARDS/ALI, arrhythmia requiring treatment, acute myocardial ischaemia, acute cardiac failure, stroke/TIA and acute kidney injury. Postoperative complications were classified according to the Clavien–Dindo systematic classification25 of postoperative morbidity and included as a binary variable (major, including IIIA to V, or minor complications, including I and II classes of the score).
Short-term oncological outcomes including final pathological stage and nodal upstaging were also compared. Regarding middle-term outcomes, the endpoints were recurrence rate and overall survival (OS) and disease-free survival (DFS). Only medically confirmed relapses were considered. OS was defined as the time between surgery and death—whatever the cause—or date of the last follow-up. The DFS corresponded to the time from surgery to tumour recurrence of the same lung cancer (local, regional or distant) or death from any cause, whichever came first, with censorship at last follow-up.
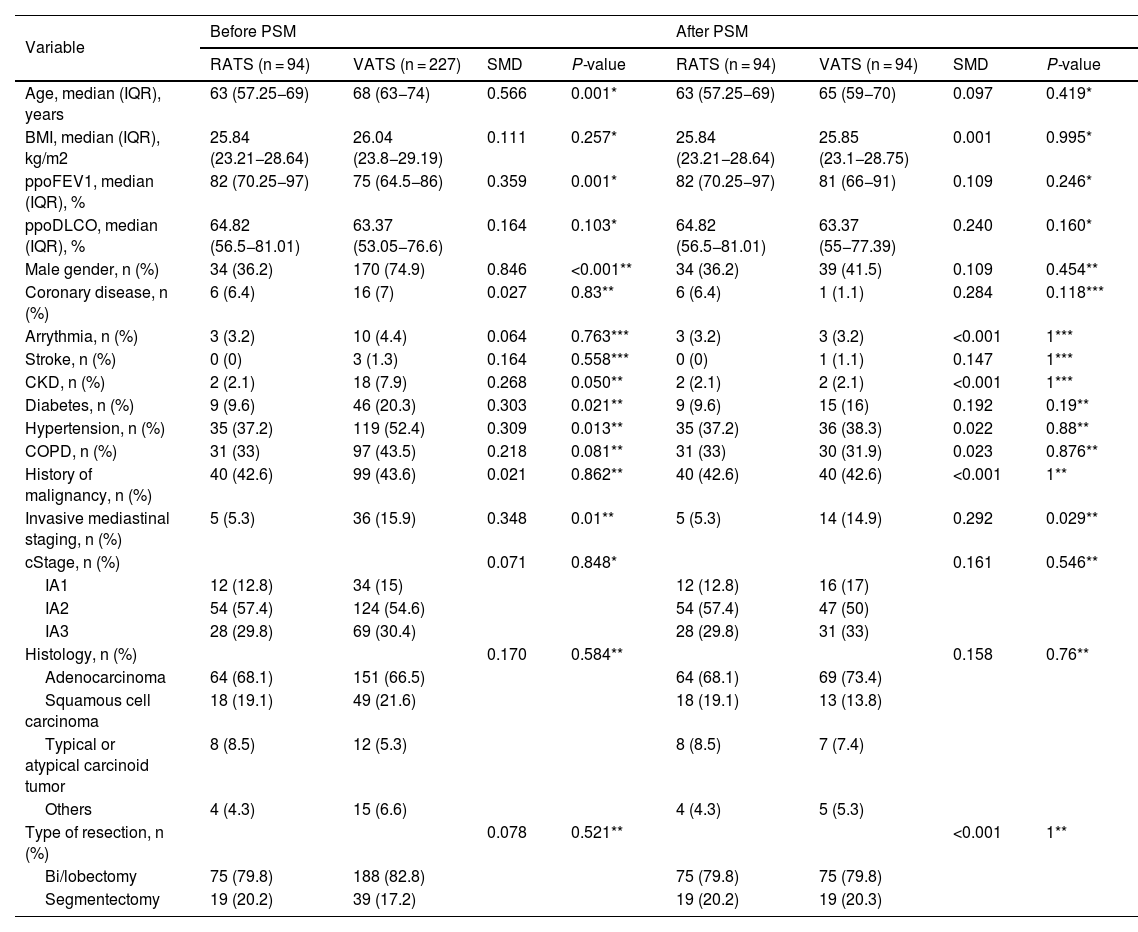
Propensity score matchingTo reduce the bias comparing outcomes among the two cohorts of patients due to confounding variables, a propensity score matching (PSM) was performed. The 1:1 nearest neighbour matching without replacement method was applied, in which each case was paired with an available control having the closest propensity score to it. Any remaining controls were left unmatched and excluded from further analysis. The distances between the samples of the 2 groups were calculated using the logistic regression. The variables used for propensity score matching were selected according to their clinical importance and statistical significance: age, sex, histology and type of resection (bi/lobectomy and anatomical segmentectomy). Covariate balance was assessed by SMD (standardised mean difference), before and after matching. SMD < 0.1 or 0.05 were considered good or excellent, respectively, to exclude residual imbalance.30
Statistical analysisDemographic characteristics of patients, clinicopathological data and perioperative outcomes in each group were analysed and compared. Categorical variables were compared using the Pearson χ2 test or Fisher’s exact test. Normally distributed continuous variables are presented as the mean standard deviation (SD), and Student’s t test was used for comparisons. For continuous variables that were not normally distributed, data are presented as the median (interquartile range [IQR]) and were compared by the Mann–Whitney U test between the groups. The test level between the two groups was set at α = 0.05 (bilateral), and a two-sided p < 0.05 was considered statistically significant.
OS and DFS rates were examined for all included patients with Kaplan–Meier curves and compared between RATS and VATS approaches with the log-rank test.
The MatchIt package of R software, v4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) was used for PSM, and SPSS software, v26.0 (SPSS Inc., Chicago, IL, USA) for further data analysis.
ResultsA total of 321 patients (94 RATS and 227 VATS cases) were included. After PSM, 94 VATS and 94 RATS cases were compared. The characteristics of the patients before and after PSM are presented in Table 1. The demographics, pulmonary function and comorbidity were similar in both groups and most of the covariates reached a good or excellent balance after matching.
Patient demographics and baseline characteristics before and after PSM.
| Variable | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| RATS (n = 94) | VATS (n = 227) | SMD | P-value | RATS (n = 94) | VATS (n = 94) | SMD | P-value | |
| Age, median (IQR), years | 63 (57.25−69) | 68 (63−74) | 0.566 | 0.001* | 63 (57.25−69) | 65 (59−70) | 0.097 | 0.419* |
| BMI, median (IQR), kg/m2 | 25.84 (23.21−28.64) | 26.04 (23.8−29.19) | 0.111 | 0.257* | 25.84 (23.21−28.64) | 25.85 (23.1−28.75) | 0.001 | 0.995* |
| ppoFEV1, median (IQR), % | 82 (70.25−97) | 75 (64.5−86) | 0.359 | 0.001* | 82 (70.25−97) | 81 (66−91) | 0.109 | 0.246* |
| ppoDLCO, median (IQR), % | 64.82 (56.5−81.01) | 63.37 (53.05−76.6) | 0.164 | 0.103* | 64.82 (56.5−81.01) | 63.37 (55−77.39) | 0.240 | 0.160* |
| Male gender, n (%) | 34 (36.2) | 170 (74.9) | 0.846 | <0.001** | 34 (36.2) | 39 (41.5) | 0.109 | 0.454** |
| Coronary disease, n (%) | 6 (6.4) | 16 (7) | 0.027 | 0.83** | 6 (6.4) | 1 (1.1) | 0.284 | 0.118*** |
| Arrythmia, n (%) | 3 (3.2) | 10 (4.4) | 0.064 | 0.763*** | 3 (3.2) | 3 (3.2) | <0.001 | 1*** |
| Stroke, n (%) | 0 (0) | 3 (1.3) | 0.164 | 0.558*** | 0 (0) | 1 (1.1) | 0.147 | 1*** |
| CKD, n (%) | 2 (2.1) | 18 (7.9) | 0.268 | 0.050** | 2 (2.1) | 2 (2.1) | <0.001 | 1*** |
| Diabetes, n (%) | 9 (9.6) | 46 (20.3) | 0.303 | 0.021** | 9 (9.6) | 15 (16) | 0.192 | 0.19** |
| Hypertension, n (%) | 35 (37.2) | 119 (52.4) | 0.309 | 0.013** | 35 (37.2) | 36 (38.3) | 0.022 | 0.88** |
| COPD, n (%) | 31 (33) | 97 (43.5) | 0.218 | 0.081** | 31 (33) | 30 (31.9) | 0.023 | 0.876** |
| History of malignancy, n (%) | 40 (42.6) | 99 (43.6) | 0.021 | 0.862** | 40 (42.6) | 40 (42.6) | <0.001 | 1** |
| Invasive mediastinal staging, n (%) | 5 (5.3) | 36 (15.9) | 0.348 | 0.01** | 5 (5.3) | 14 (14.9) | 0.292 | 0.029** |
| cStage, n (%) | 0.071 | 0.848* | 0.161 | 0.546** | ||||
| IA1 | 12 (12.8) | 34 (15) | 12 (12.8) | 16 (17) | ||||
| IA2 | 54 (57.4) | 124 (54.6) | 54 (57.4) | 47 (50) | ||||
| IA3 | 28 (29.8) | 69 (30.4) | 28 (29.8) | 31 (33) | ||||
| Histology, n (%) | 0.170 | 0.584** | 0.158 | 0.76** | ||||
| Adenocarcinoma | 64 (68.1) | 151 (66.5) | 64 (68.1) | 69 (73.4) | ||||
| Squamous cell carcinoma | 18 (19.1) | 49 (21.6) | 18 (19.1) | 13 (13.8) | ||||
| Typical or atypical carcinoid tumor | 8 (8.5) | 12 (5.3) | 8 (8.5) | 7 (7.4) | ||||
| Others | 4 (4.3) | 15 (6.6) | 4 (4.3) | 5 (5.3) | ||||
| Type of resection, n (%) | 0.078 | 0.521** | <0.001 | 1** | ||||
| Bi/lobectomy | 75 (79.8) | 188 (82.8) | 75 (79.8) | 75 (79.8) | ||||
| Segmentectomy | 19 (20.2) | 39 (17.2) | 19 (20.2) | 19 (20.3) | ||||
IQR: interquartile range; BMI: body mass index; ppoFEV1: predicted postoperative forced expiratory volume in one second; predicted postoperative carbon monoxide lung diffusion capacity; CKD: chronic kidney disease; COPD: Chronic obstructive pulmonary disease.
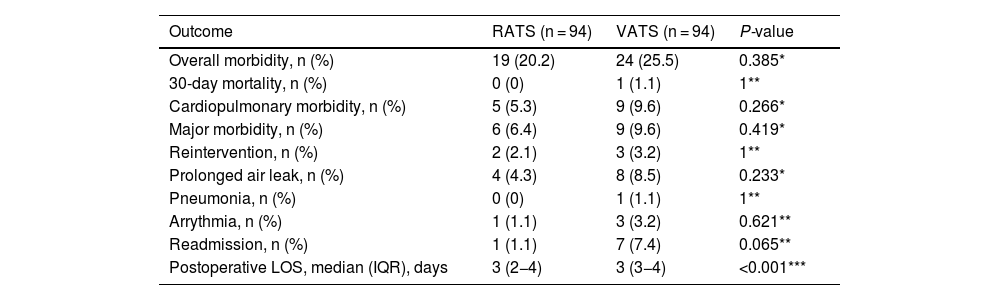
The data for the perioperative outcomes of the RATS and VATS matched groups are listed in Table 2. There was no operative mortality. Thirty-day or in-hospital mortality was nil in the RATS group and 1.1% in the VATS group, overall morbidity was comparable among RATS and VATS cases (20.2% vs 25.5%, P = 0.385, respectively). No differences among groups were found on cardiopulmonary morbidity, major complications and the commonest postoperative complications. Postoperative length of hospital stay (LOS) was significantly shorter in the RATS group compared to that of VATS cases (P < 0.001).
Perioperative outcomes of RATS and VATS after PSM.
| Outcome | RATS (n = 94) | VATS (n = 94) | P-value |
|---|---|---|---|
| Overall morbidity, n (%) | 19 (20.2) | 24 (25.5) | 0.385* |
| 30-day mortality, n (%) | 0 (0) | 1 (1.1) | 1** |
| Cardiopulmonary morbidity, n (%) | 5 (5.3) | 9 (9.6) | 0.266* |
| Major morbidity, n (%) | 6 (6.4) | 9 (9.6) | 0.419* |
| Reintervention, n (%) | 2 (2.1) | 3 (3.2) | 1** |
| Prolonged air leak, n (%) | 4 (4.3) | 8 (8.5) | 0.233* |
| Pneumonia, n (%) | 0 (0) | 1 (1.1) | 1** |
| Arrythmia, n (%) | 1 (1.1) | 3 (3.2) | 0.621** |
| Readmission, n (%) | 1 (1.1) | 7 (7.4) | 0.065** |
| Postoperative LOS, median (IQR), days | 3 (2−4) | 3 (3−4) | <0.001*** |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; LOS: length of hospital stay.
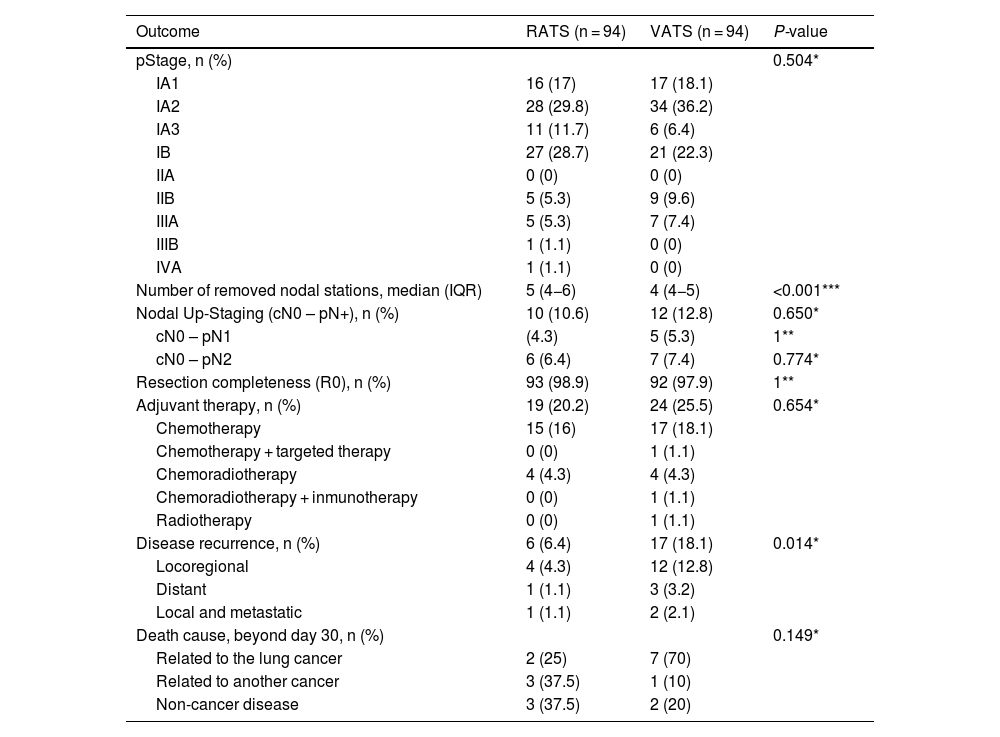
87.2% of cases undergoing RATS anatomical resection were finally categorized as pathological stage I, while 83% of VATS cases were finally diagnosed as pathological stage I. A higher number of nodal stations was removed though RATS approach. Pathological N1/N2 upstaging was similar in both groups (10.6% in RATS and 12.8% in VATS, P = 0.65). Six cases without nodal involvement were categorized as T3 due to the presence of PL3 (3 in the VATS group and 1 in the RATS) and the presence of two nodules in the same lobe (one in the VATS group and 2 in the RATS group). Resection completeness (R0) was similar in both groups (98.9% in the RATS and 97.9% in VATS, P = 1). Postoperative adjuvant therapy was administered in 20.2% of RATS cases and 25.5% of VATS patients, P = 0.385. In patients without lymph node involvement, the main reason for indicating adjuvant chemotherapy was pleural invasion which was found in 9.6% of all RATS cases and 14.9% of VATS series (P = 0.266). During the 3.5-year follow-up period (median: 29 months; IQR: 18–39), recurrence rate was 6.4% in RATS group; meanwhile, in the VATS group, recurrence was observed in 18.1% (P = 0.014). Details are shown in Table 3.
Oncological outcomes of RATS and VATS after PSM.
| Outcome | RATS (n = 94) | VATS (n = 94) | P-value |
|---|---|---|---|
| pStage, n (%) | 0.504* | ||
| IA1 | 16 (17) | 17 (18.1) | |
| IA2 | 28 (29.8) | 34 (36.2) | |
| IA3 | 11 (11.7) | 6 (6.4) | |
| IB | 27 (28.7) | 21 (22.3) | |
| IIA | 0 (0) | 0 (0) | |
| IIB | 5 (5.3) | 9 (9.6) | |
| IIIA | 5 (5.3) | 7 (7.4) | |
| IIIB | 1 (1.1) | 0 (0) | |
| IVA | 1 (1.1) | 0 (0) | |
| Number of removed nodal stations, median (IQR) | 5 (4−6) | 4 (4−5) | <0.001*** |
| Nodal Up-Staging (cN0 – pN+), n (%) | 10 (10.6) | 12 (12.8) | 0.650* |
| cN0 – pN1 | (4.3) | 5 (5.3) | 1** |
| cN0 – pN2 | 6 (6.4) | 7 (7.4) | 0.774* |
| Resection completeness (R0), n (%) | 93 (98.9) | 92 (97.9) | 1** |
| Adjuvant therapy, n (%) | 19 (20.2) | 24 (25.5) | 0.654* |
| Chemotherapy | 15 (16) | 17 (18.1) | |
| Chemotherapy + targeted therapy | 0 (0) | 1 (1.1) | |
| Chemoradiotherapy | 4 (4.3) | 4 (4.3) | |
| Chemoradiotherapy + inmunotherapy | 0 (0) | 1 (1.1) | |
| Radiotherapy | 0 (0) | 1 (1.1) | |
| Disease recurrence, n (%) | 6 (6.4) | 17 (18.1) | 0.014* |
| Locoregional | 4 (4.3) | 12 (12.8) | |
| Distant | 1 (1.1) | 3 (3.2) | |
| Local and metastatic | 1 (1.1) | 2 (2.1) | |
| Death cause, beyond day 30, n (%) | 0.149* | ||
| Related to the lung cancer | 2 (25) | 7 (70) | |
| Related to another cancer | 3 (37.5) | 1 (10) | |
| Non-cancer disease | 3 (37.5) | 2 (20) |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery.
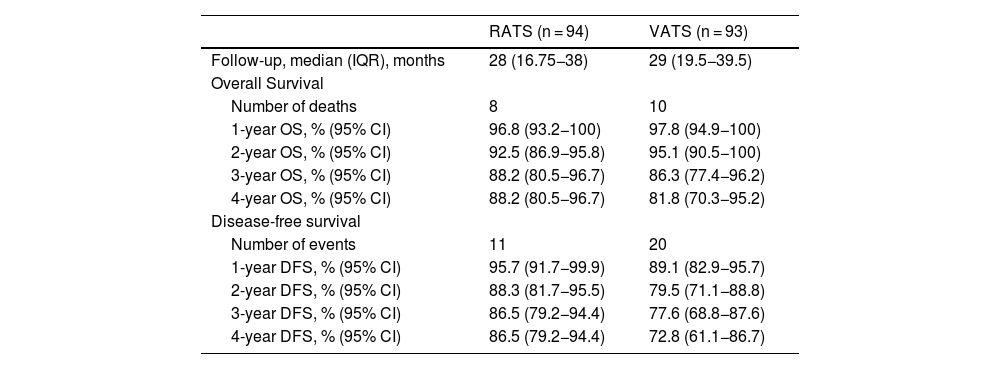
After propensity-score matching, OS and DFS were similar in RATS and VATS groups (log rank P = 0.848 and log rank P = 0.117, respectively) (Fig. 1). The 5-year OS and DFS of patients who had undergone anatomical lung resection by RATS or VATS could not be estimated, but the 4-year OS and DFS were estimated at 88.2% (80.5−96.7) and 86.5% (79.2−94.4), respectively in the RATS group and at 81.8% (70.3−95.2) and 72.8% (61.1−86.7), respectively in the VATS group. Details are shown in Table 4.
Middle-term survival of patients surviving beyond day 30 after surgery, according to VATS and RATS procedure.
| RATS (n = 94) | VATS (n = 93) | |
|---|---|---|
| Follow-up, median (IQR), months | 28 (16.75−38) | 29 (19.5−39.5) |
| Overall Survival | ||
| Number of deaths | 8 | 10 |
| 1-year OS, % (95% CI) | 96.8 (93.2−100) | 97.8 (94.9−100) |
| 2-year OS, % (95% CI) | 92.5 (86.9−95.8) | 95.1 (90.5−100) |
| 3-year OS, % (95% CI) | 88.2 (80.5−96.7) | 86.3 (77.4−96.2) |
| 4-year OS, % (95% CI) | 88.2 (80.5−96.7) | 81.8 (70.3−95.2) |
| Disease-free survival | ||
| Number of events | 11 | 20 |
| 1-year DFS, % (95% CI) | 95.7 (91.7−99.9) | 89.1 (82.9−95.7) |
| 2-year DFS, % (95% CI) | 88.3 (81.7−95.5) | 79.5 (71.1−88.8) |
| 3-year DFS, % (95% CI) | 86.5 (79.2−94.4) | 77.6 (68.8−87.6) |
| 4-year DFS, % (95% CI) | 86.5 (79.2−94.4) | 72.8 (61.1−86.7) |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; IQR: interquartile range; CI: confidence interval.
Controversy still exists about the optimal surgical approach to early-stage NSCLC. Ideally, the chosen approach should be safe and minimize patient postoperative morbidity to enhance recovery and maximize oncologic efficacy in order to obtain optimal long-term outcomes.
Our study compared the two currently used minimally invasive approaches focusing not only on the short-term but also, on the middle-term oncological outcomes in patients with clinical stage IA NSCLC using data collected from a single high-volume referral centre. Furthermore, propensity matched analysis potentially reduced heterogeneities between populations and allowed comparison between similar patients.
Our results demonstrated that RATS, a recently developed surgical approach, can be performed safely in comparison to currently mature and well-known VATS approach, providing similar OS and DFS in clinical stage I NSCLC patients. Moreover, we found that the cumulative recurrence rate was significantly lower in the RATS group compared to VATS.
Our findings corroborate the results of different studies focused on evaluating feasibility and safety of robotic anatomical lung resection compared to VATS approach. In 2017 a systematic-review and meta-analysis conducted by Wei et al.31 demonstrated that RATS lobectomy was a feasible and safe technique and could achieve equivalent short-term outcomes compared to VATS. Another recently published meta-analysis came to the same conclusion indicating that perioperative outcomes of RATS and VATS for NSCLC are equivalent.12,14–17,32 In the current study, we demonstrated the statistically significant advantage of RATS over VATS in terms of length of hospital stay as it has been also proven in two of the previously mentioned meta-analysis,14,17 although the difference found was not clinically relevant (median LOS: 3 days in both groups).
To assess the oncologic effectiveness of robotic approach in clinical stage IA NSCLC, we evaluated the prevalence of nodal upstaging in both groups, and no differences were found among RATS and VATS approach. Similar results were published by Lee et al.33 who retrospectively reviewed clinically node-negative patients with lung cancer undergoing VATS or robotic lobectomy showing no difference in pN1 and pN2 upstaging in cT1 tumours between VATS and RATS (6.7% vs. 7.5% and 5.9% vs. 5%, respectively; P = 0.97). Nevertheless, in the current study, a higher number of nodal stations was removed through RATS approach (P < 0.001). We speculate that this difference was attributable to the higher capability of RATS in dissecting lymph nodes. In our experience, the robotic system allows the surgeon to dissect around smaller vessels, bronchi, and surrounding lymph nodes more efficiently than VATS due to the wrist-like action of the robotic instruments conferring superiority in this regard.
Nonetheless, we found an incidence of locoregional recurrence of 5.3% (5/94) in the RATS group, versus 14.9% (14/94) in the VATS one within the follow-up period of 29 months. Several factors such as tumour size, resection margin and lymph node dissection may influence the occurrence of local recurrence in resected early-stage NSCLC. In the current study, a higher rate of VATS cases was finally staged as IIB-IIIA and require adjuvant therapy, although no significant differences were found regarding resection completeness. Additionally, RATS approach achieved a higher number of nodal stations removed, although nodal upstaging was slightly higher in the VATS group. These results could be explained by the fact that pathological upstaging, even if it is related to the number of lymph nodes removed, it is also determined by the real N2 involvement, which for stage I NSCLC is directly related to the aggressiveness of the tumour. All these facts considered together could explain the difference in locoregional incidence among both groups.
However, OS and DFS showed no statistical difference when the two groups were compared. Similarly, Casiraghi et al.23 in a matched analysis comparing long-term outcomes of RATS, VATS and open surgery in stage I NSCLC patients found a higher incidence of recurrences, in particular local recurrences, in VATS compared to RATS and open surgery. Like us, they did not found differences in OS and cancer-specific survival between VATS and RATS. Furthermore, in 2017, Yang et al.20 conducted a propensity-score matched analysis study to compare long-term outcomes among RATS, VATS, and open lobectomy for clinical stage I NSCLC patients, showing that the 5-year OS for RATS, VATS and open surgery was 77.6%, 73.5% and 77.9%, respectively, without a statistically significant difference. However, 5-year DFS rates were 72.7%, 65.5%, and 69.0%, respectively, with a statistically significant difference between the robotic and VATS groups (P = 0.047).
Our study presents several limitations. First, although individual matching decreased selection bias among groups, our study is limited by its retrospective nature. Furthermore, the variables used for PSM (age, sex, histology, and type of resection) were selected according to their clinical importance regarding especially middle-term outcomes. Nonetheless, the distribution of other confounder variables mainly influencing short-term outcomes was homogeneous among RATS and VATS groups. Secondly, RATS series includes patients who underwent surgery during the learning curve, when patients were carefully selected leading to a higher prevalence of female cases and a lower need for invasive staging. Additionally, data were obtained from a single public institution and the sample size was limited. Therefore, our results should be confirmed in additional patient cohorts from different multi-institutional databases and longer follow-up. Thirdly, we did not analyse the total number of lymph nodes removed which is considered one of the main ways to assess the effectiveness of NSCLC surgery. Instead, we measured oncological outcomes in terms of nodal upstaging, recurrence incidence and OS and DFS, which allowed us to understand the possible differences between approaches.
ConclusionRATS can be performed safely in patients with early-stage NSCLC. For clinical stage IA disease, robotic anatomic lung resection offers better oncologic outcomes in terms of recurrence, although there are no differences in OS and DFS compared with VATS.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interest directly or indirectly related to the contents of the manuscript.