Serrated polyposis syndrome (SPS) is a rare entity characterized by the presence of multiple hyperplastic polyps in the colon and an increased risk of presentation and development of colorectal cancer (CRC).
ObjectiveTo evaluate the clinical and phenotypical characteristics of patients who present one of the 3 WHO criteria for the diagnosis of SPS diagnosed and treated at a tour hospital.
Patients and methodsPatients with the diagnosis of SPS during 2005–2012 were revised; 24.208 colonoscopies were performed during this period. Other entities include age, sex, family history of CRC (APC/MYH), proximal/mixed/distal phenotype, indication for colonoscopy, number, size, location of the hyperplastic polyps, presence of mixed/adenomatous polyps, CRCI, follow-up and endoscopio/surgical treatment.
ResultsA total of 23 cases were included (19 male). The median age was 51. 34% of the cases had a prior family history of CRC or polpyps. Distal phenotype was more frequent (48%). Another 73% presented synchronous adenomatous polyps, and 26% a CRC. 57% of them were asymptomatic. Surgery was performed in 9 cases (6 for cancer and 3 for polyposis), and 14 were treated by polypectomy and observation. Eleven patients (47%) presented recurrent/persistent lesions after initial surgical/endoscopic treatment.
ConclusionSPS is a heterogeneous syndrome that is variable in the type, size, distribution and number of polyps, and is more common in male smokers with a distal phenotype. The majority of patients also present synchronous adenomatous polyps. These patients require an organized multidisciplinary evaluation.
El síndrome de poliposis serrada (SPS) es una entidad rara caracterizada por la presencia de múltiples pólipos de histología hiperplásica en el colon y un riesgo aumentado de presentar y desarrollar cáncer colorrectal (CCR).
ObjetivoEvaluar las características clínicas y fenotípicas de los sujetos que reúnen alguno de los 3 criterios de la OMS para el diagnóstico de SPS, diagnosticados y seguidos en nuestro hospital.
Pacientes y métodosSe revisan los pacientes con SPS durante 2005-2012, periodo en el que se realizan 24.208 colonoscopias. Se analizan edad, sexo, historia familiar de CCR (APC/MYH), fenotipo proximal/mixto/distal, indicación de colonoscopia, número, tamaño, localización de los pólipos hiperplásicos, presencia de pólipos mixtos/adenomatosos, CCRI, seguimiento y tratamiento endoscópico/quirúrgico.
ResultadosSe han recogido 23 casos (19 hombres). El promedio de edad fue 51 años. El 34% presentaba antecedentes familiares de CCR o pólipos. El fenotipo distal (48%) fue más frecuente. El 73% presentaba pólipos adenomatosos sincrónicamente, y el 26% un CCR. El 57% eran pacientes asintomáticos. Se realizó cirugía en 9 casos (6 por cáncer y 3 por poliposis, y 14 con polipectomías sucesivas y observación). Un total de 11 pacientes (47%) presentaron lesiones recurrentes/persistentes tras el tratamiento quirúrgico/endoscópico inicial.
ConclusiónEl SPS es un síndrome heterogéneo, variable en tipo, tamaño, distribución y número de pólipos, siendo más frecuente en varones fumadores con fenotipo distal. La mayoría de los pacientes presentan además pólipos adenomatosos de manera sincrónica. Estos pacientes requieren una evaluación organizada multidisciplinar.
Colorectal cancer (CRC) affects 6% of the general population. Incidence forecasts for 2015 indicate that 30230 people in Spain will be affected making it the most frequent type of cancer, far more than lung and breast cancer.1 Currently, it is the second cause of cancer-related mortality in Spain,2 accounting for 12.7% of deaths in males and 15% in females.3
The adenoma–carcinoma sequence is considered the main pathway for colorectal carcinogenesis. Adenomatous polyps can evolve into a malignancy through the “traditional” pathway of carcinogenesis, namely characterized by loss of heterozygosity or APC and P53 gene allelic loss and the resulting chromosomal imbalance. This is the carcinogenesis pathway for 70%–80% of CRC.4,5
Hyperplastic polyps are presumed to be benign, malignancy-free lesions; however, in the last 2 decades, subtypes of these polyps have been reported. Today, they are known collectively as serrated polyps, due to the “saw-toothed” infolding of the crypt epithelium, which do pose a cancer risk. Two serrated pathway subtypes are described6,7: (a) sessile serrated adenoma through the serrated pathway; it occurs typically in the proximal colon, and its initial molecular change is MAP kinase signalling pathway activation by BRAF proto-oncogene mutation leading to CpG island methylator phenotype (CIMP-high); this is defined by methylation and subsequent silencing of the tumour gene promoter region, such as MLH1, and ensuing microsatellite instability (MSI); (b) the “alternate serrated pathway,” likely through traditional serrated adenoma, although less well defined than the former and characterized by KRAS gene mutation, leading to low-level methylator phenotype (CIMP-low) and silencing of the MGMT promoter region. Serrated pathways may be the cause of up to 30% of CRC.6,7
Hyperplastic polyposis syndrome, or rather, serrated polyposis syndrome (SPS) is rare and poorly understood entity; its genetic base is unknown, although it shows characteristics distinctive of diseases with genetic predisposition8–10 such as colonic serrated polyps, early diagnosis, and greater prevalence conferred to CRC family history. Patients affected by this syndrome carry a high risk (25%–40%) of developing CRC10–13; sporadic as well as hereditary cases have been reported.9–11 A great rate of extracolonic cancer seems to exist.14–16 SPS likely includes a variety of patient types having different phenotypes; however, to this date, no major phenotype-related risk of developing CRC has been described.15,16
This study aims to assess the phenotype and clinical characteristics of patients meeting SPS criteria.
Materials and MethodsTable 1 lists the clinical criteria for SPS diagnosis reported by Burt and Jass and approved by the WHO in 200017; they were redefined in Berlin in 2010.18 SPS symptom diagnosis requires meeting one of the 3 criteria.
WHO Criteria for SPS Diagnosis.
| 1. At least 5 polyps proximal to the sigmoid colon; 2 of them are equal to or larger than 10mm |
| 2. Any number of polyps proximal to the sigmoid colon in an individual with family history of HPS first degree relative |
| 3. More than 20 serrated polyps of any size distributed along the colon |
WHO: World Health Organization; SPS: Serrated Polyposis Syndrome.
Patients diagnosed with SPS between 2005 and 2012 were examined retrospectively for this study. During this period, 24208 colonoscopies were performed at the hospital on asymptomatic patients from the blood-in-stools screening system population programme carried out in our community, and also on at-risk patients with a family history of CRC and on symptomatic patients (haematochezia, abdominal pain or bowel habit changes).
A total of 23 patients diagnosed with SPS met WHO criteria and were included in our study.
To determine the various phenotype characteristics of these patients, we analyzed their demographic data (age, gender, time of diagnosis), clinical data (symptoms, CRC or SPS family history, smoking habit, other associated neoplasia), endoscopy data (number, location, size of polyps, presence of synchronous adenomatous polyps or CRC), phenotype pattern data (proximal to the splenic flexure), distal (descending, sigmoid and rectal colon) or mixed (mostly located on the left side, however having more than 3 polyps of any size or one larger than 1cm on the right side), and pathological data (HP, sessile serrated adenoma, traditional serrated adenoma and carcinoma). Some patients underwent genetic testing to rule out other APC or MYH gene-associated polyposis. Finally, endoscopic, surgical treatment and follow-up were recorded.
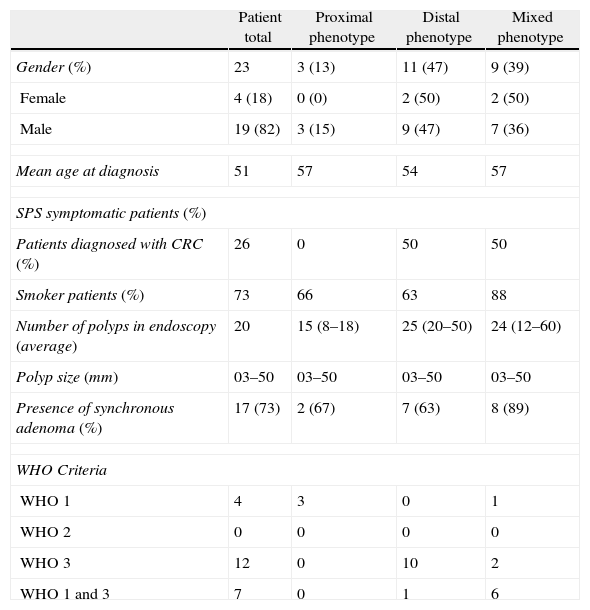
ResultsWe found 23 cases that met one of the 3 WHO criteria on SPS clinical diagnosis. Table 2 shows epidemiological, clinical and phenotypical characteristics for the series of patients with SPS.
Clinical and Endoscopic Characteristics of SPS Patients.
| Patient total | Proximal phenotype | Distal phenotype | Mixed phenotype | |
| Gender (%) | 23 | 3 (13) | 11 (47) | 9 (39) |
| Female | 4 (18) | 0 (0) | 2 (50) | 2 (50) |
| Male | 19 (82) | 3 (15) | 9 (47) | 7 (36) |
| Mean age at diagnosis | 51 | 57 | 54 | 57 |
| SPS symptomatic patients (%) | ||||
| Patients diagnosed with CRC (%) | 26 | 0 | 50 | 50 |
| Smoker patients (%) | 73 | 66 | 63 | 88 |
| Number of polyps in endoscopy (average) | 20 | 15 (8–18) | 25 (20–50) | 24 (12–60) |
| Polyp size (mm) | 03–50 | 03–50 | 03–50 | 03–50 |
| Presence of synchronous adenoma (%) | 17 (73) | 2 (67) | 7 (63) | 8 (89) |
| WHO Criteria | ||||
| WHO 1 | 4 | 3 | 0 | 1 |
| WHO 2 | 0 | 0 | 0 | 0 |
| WHO 3 | 12 | 0 | 10 | 2 |
| WHO 1 and 3 | 7 | 0 | 1 | 6 |
SPS: Serrated Polyposis Syndrome; WHO: World Health Organization.
Mean age of diagnosis was 51 years (18–80 years). A total of 19 (82%) of the 23 patients were male; age differences were ignored, as they met one or some WHO or phenotype criteria. All patients met diagnosis criterion 1 or 3 (or both); none met criterion 2 (first-degree relatives with SPS). As expected, the 3 phenotype patterns corresponding to the location of serrated polyps in the colon were correlated to WHO diagnostic definitions, and their relative frequencies are listed in the results table, including how polyp location, number and size relate to phenotype.
Colonoscopies showed an annual incidence of SPS of 0.08%. Thirteen (57%) of the diagnosed patients were asymptomatic and diagnosed either after population screening or due to family history of colorectal neoplasia. Ten patients (43%) had symptoms requiring colonoscopy.
Six male patients (26%) were diagnosed with colorectal cancer synchronous to SPS. Half of these patients had distal phenotype and the other half had mixed phenotype. The left colon was the most common location (4 cases). 66% of CRC patients showed symptoms, compared to 33% of non-CRC patients with SPS (and distal or mixed phenotype) who also had symptoms. Incidence in smokers was rather high, including CRC patients (100%) and non-CRC patients with SPS (73%). Four patients had extracolonic cancer from various origins (parotid, endometrium, urothelial and oesophageal tumour); three of these patients also had synchronous colonic adenoma, and none had associated CRC.
Initially, all SP patients were diagnosed histologically as HP, and no subtypes were specified. Some of these lesions were reclassified only after examining surgical samples and conducting pathologist meetings. Traditional serrated adenoma, which is more frequent in mixed or distal phenotype patients, presented fewer problems in being identified than the distinction between hyperplastic polyp and sessile serrated adenoma, which is more elusive. Furthermore, no specific microscopic alterations were identified in SPS-associated carcinoma in terms of “sporadic” carcinoma.
A total of 73% of SPS patients had SP-synchronous adenomatous polyps. The studied cases show a greater relationship between mixed phenotype and synchronous adenoma. A genetic study of APC and MYH was conducted on 5 patients with attenuated polyposis to rule out MYH-associated adenomatous polyposis or recessive polyposis. All SPS cases with CRC also showed synchronous colonic adenoma (average of 3–4 adenoma).
Surgery was needed in 9 cases (39%) because colonoscopy failed to resect all the polyps. Five subtotal colectomies were performed, 2 right colectomies, one right hemicolectomy with synchronous sigmoidectomy (due to double tumour) and a left colectomy expanded to the transverse colon. All other patients were treated by endoscopic polypectomies and follow-up.
During the follow-up on patients who underwent surgery, one patient who had a previous sigmoid carcinoma developed rectal metachronous T1 carcinoma that required local excision by transanal endoscopic operation (TEO); new rectal HPs were excised endoscopically in 2 other cases. Eight polyps (57%) in 14 patients followed with endoscopy needed polypectomies due to SP persistence or recurrence (none larger than 10mm), including 3 of these patients with synchronous tubular adenoma.
DiscussionSPS diagnosis criteria, although agreed by consensus,17,18 are quantitatively arbitrary and possibly restrictive, and this may lead to infradiagnosis of the disease. Frequently, these polyps are overlooked in a colonoscopy,19 due to the small size, non-threatening morphology and poor count, added to the difficulty of finding and classifying the various anatomopathological serrated lesion subtypes.20 SPS annual prevalence was 0.08% in this case series. This figure matches that of other case series which include symptomatic and screening cases, similar to ours.19 However, it is difficult to know if this incidence is representative of the general population since participation in screening is not accepted by everyone, and the fact that colonoscopies also include at-risk patients, who may over represent general incidence.
SPS in this study was more frequent in males, although other case series failed to show variation between both sexes11,14,15; the age ranges between 18 and 80 years (mean age: 51 years, as in this case series), and there were no differences between SPS phenotypes. The most frequent SPS phenotypes we found were: distal (multiple small size polyps), and mixed (numerous polyps on the left side associated with few larger size polyps on the right side or, less frequently, with various polyps on the right side and smaller on the left side). Proximal phenotype was the least frequent, showing a polyp pattern characterized by being fewer in number yet larger in size, where serrated sessile adenoma was more relevant.
Overall, we found a 26% synchronous CRC prevalence for SPS cases, in line with the literature (18–40%).9,10,21 Our case series is limited to relating phenotypes with their risk of having or developing CRC. Although in this study we failed to find proximal phenotype-associated synchronous CRC, there is evidence that serrated lesions larger than 10mm (in particular, sessile adenoma) are a risk factor for developing or having proximal CRC,22,23 which is not the case for lesions smaller than 5mm.23
The extracolonic cancer prevalence that we found (17%) in this case series is similar to that in other reports, despite the fact that none had synchronous CRC, which is described in the literature.11,16
Although we found synchronous adenomatous polyps in the 3 phenotypes, a greater amount of coincident adenoma is found in the mixed phenotype. These figures match those of previously published studies reporting percentages of adenoma presence between 69 and 88%.9–11,15
The SPS hereditary pattern is unknown; however, compatibility with autosomal recessive inheritance has been indicated,12,24,25 even though some environmental factors, such as smoking,21 could modulate SPS phenotypical differences through hypermethylation of tumour-suppressor gene promoters, and polyp characteristics would differ depending on BRAF or KRAS mutation.8,13,15,24,25 Small polyps in the distal colon seem to be related to KRAS mutation; larger polyps in the proximal colon seem to be related to BRAF mutation.8,24,25 Considering mixed phenotype patients, both mutations may coexist in some cases.
The frequent existence of adenomatous polyps in SPS patients makes it harder to differentiate attenuated adenomatous polyposis from SPS. When polyposis is found, an expert pathologist must perform several polypectomies/biopsies in various locations of the colon for histological analysis and correct characterization. Communication with pathologists is fundamental, keeping in mind the difficulties we found to histologically identify the various subtypes of serrated lesions. Even if the immediate family has no history of this condition or when in doubt, a genetic analysis of the APC gene must be performed, since family involvement is well-known, and because of the implications it entails for therapy. Detecting the presence of mutated KRAS or BRAF can also be useful, as well as an MYH analysis, although it is harder to perform.26 Patients with recessive adenomatous polyposis due to mutations of MYH also carried a high risk of colorectal and extracolonic cancer.27 These analyses are also relevant for prophylactic colectomy.
SPS surgical or endoscopic treatment must be suitable for the patient's phenotype. Full treatment and follow-up of SPS patients by colonoscopy are possible,27 although surgery has been needed in more than a third of cases, due to the impossibility of excision and monitoring. Total excision of lesions is recommended, in particular for those larger than 5mm and located in the proximal colon; biopsy or fulguration is sufficient for lesions smaller than 5mm and located in the rectum.28 Surgery would be indicated for large-size polyps that cannot be excised by colonoscopy or due to their quantity. Despite increased CRC incidence, we do not think that subtotal colectomy is justified for all SPS cases requiring surgery. Right colectomy would be justified when right colon lesions, even with rectal or sigmoid HP, cannot be excised, as long as proper follow-up can be performed. Furthermore, mixed phenotype may indeed require subtotal colectomy to control a large number of polyps distributed throughout the colon. Distal phenotypes that cannot be managed endoscopically, but having excised right polyps, may be treated by left colectomy and subsequent follow-up.
The risk of metachronous lesion implies planning an endoscopic follow-up, which should be carried out at shorter intervals than those after polyp excision or sporadic cancer resection because serrated pathway carcinogenesis seems faster than conventional pathway carcinogenesis.15,19 An annual colonoscopy for an indefinite time is recommended for SPS patient follow-up.28–30 Colonoscopy is recommended every 5 years for first degree relatives (regardless of the presence of CRC) starting at 40–55 years or 5 years before the age of diagnosis of the affected relative.28–30
In summary, SPS is a heterogeneous syndrome that varies in type, size, distribution and number of polyps and is more frequent in male smokers with a distal phenotype. Additionally, most patients have synchronous adenomatous polyps. These patients require a multidisciplinary assessment approach.
Conflict of InterestThe authors declare having no conflict of interest.
Please cite this article as: Elorza G, Enríquez-Navascués JM, Bujanda L, Larzábal M, Gil Lasa I, Martí L. Características fenotípicas de los pacientes con síndrome de poliposis serrada de colon: estudio de 23 casos. Cir Esp. 2014;92:659–664.