Surgical site infection is one of the most prevalent healthcare-associated infections and presents a considerable morbidity. The aim of this comprehensive narrative review is to describe the evidence and grade of recommendation of the preventive measures developed in the three phases of the surgical process (preoperative, perioperative and postoperative phases), as well as coincidences and divergences between selected Clinical Practice Guidelines (CPG). Four preventive measures were recommended with similar high grade evidence in all CPG: Hair removal, antibiotic prophylaxis, surgical site preparation and normothermia. However, critical points, new preventive measures and bundle implementations by surgical process are under discussion. These results represent a significant progress toward improving programs to prevent surgical site infection and they should be taken into account for improved future interventions in this area.
La infección de sitio quirúrgico es la infección relacionada con la asistencia sanitaria más prevalente en el entorno sanitario y con una considerable morbilidad. El objetivo de esta exhaustiva revisión narrativa es describir la evidencia y el grado de recomendación de las medidas preventivas desarrolladas en las 3 fases asistenciales del enfermo quirúrgico (preoperatoria, perioperatoria y postoperatoria), así como las coincidencias y divergencias entre las guías de práctica clínica (GPC) seleccionadas. Cuatro de las medidas preventivas fueron recomendadas con similar alto grado de evidencia en todas las GPC: eliminación adecuada del vello, profilaxis antibiótica, preparación del campo quirúrgico y normotermia. Sin embargo, permanecen en debate los puntos críticos de cada intervención, las nuevas medidas preventivas surgidas y su agrupación en paquetes por procedimientos quirúrgicos. Estos resultados representan un progreso significativo de mejora en programas preventivos de las infecciones quirúrgicas y deberían tenerse en cuenta para implementar futuras intervenciones en esta área.
Healthcare-associated infections (HAI) are defined as infections that showed no evidence of their presence or incubation upon admittance to hospital, and whose origin was most likely the medical activity itself as a result of an adverse reaction to the presence of an infectious agent or toxin.1 Surgical site infections (SSI) are a type of HAI that occurs after a surgical intervention in an area of the body where the operation was carried out. SSI may involve the skin, tissues and organs or implanted material, and they are revealed by a combination of signs and symptoms.2 According to EPINE 2015 (Study of the Prevalence of Nosocomial Infection), the total rates of HAI and SSI in Spain are 8.92% and 2.29%, respectively.3
SSI occupy a prominent place in the vigilance and control of nosocomial infections,4 as their characteristics make their prevention a priority: high prevalence,3 demonstrated severity,5 great increase in direct and indirect healthcare costs6 and availability of scientifically proven effective prevention measures7,8 for each type of surgical procedure.9
Studies on the costs caused by SSI show additional costs of 14,266.80 euros per patient that develops SSI compared to patients without SSI in prosthesis surgery,10 increased mortality,11 or the economic costs of adverse events, where each SSI obtained a cost that oscillated between 1174 and 21392 dollars.12
There is a general consensus that up to 60% of SSI would be avoided by applying adequate prevention programs6,8,13 and verifying their compliance,14 since sets of measures (or “bundles”) have demonstrated a reduction in SSI rates.15–17 These results, however, can vary according to various factors, including the choice of the individual measures that constitute them.
In Spain, there is formal implementation of the most classic measures for SSI prevention. Antibiotic prophylaxis, for instance, continues to be one of the most effective measures,18 even though one out of every 4 antibiotic prophylaxes is considered inappropriate.19 In a Cochrane review, other measures have shown a 46% rate of preventive efficacy, such as the use of electric clippers and not a metal razor to eliminate hair.20
The purpose of this study is to describe the evidence given in the most updated clinical practice guides (CPG) on preventive measures to prevent SSI, considering all phases of the surgical process.
MethodsA thorough, narrative review of the literature was carried out through PubMed and other information sources: Tripdatabase and the National Guideline Clearinghouse (NGC). In addition, the International Network of Agencies for Health Technology Assessment (INAHTA) platform was consulted. We also reviewed the websites of agencies not included in INAHTA and international institutions: Centers for Disease Control and Prevention (CDC), European Center for Disease Prevention and Control (ECDC), The Cochrane Library, the platform of The Healthcare Infection Control Practices Advisory Committee, The National Institute of Health and Clinical Excellence, The Canadian Patient Safety Institute, The Society for Healthcare Epidemiology of America, the Infectious Diseases Society of America, Association for Professionals in Infection Control and Epidemiology, the American Hospital Association, the Joint Commission and The National Health Services of Scotland.
For the bibliographic search, MeSH terminology was used in the following search strategy: [(surgical wound infection OR surgical site infection) AND (prevention and control)]. The inclusion criteria were: (1) the document was categorized as CPG; (2) it included SSI prevention measures in the 3 phases of the surgical process (preoperative, peri/intraoperative, postoperative); (3) the date of publication was between January 1, 2010 and July 1, 2017; and (4) the language of publication was English or Spanish.
The bibliographic search was done by a single researcher. Duplicates were eliminated. Two independent researchers reviewed the selected documents and determined whether they met inclusion criteria. In cases where there was no consensus, a third researcher intervened.
Some of the guidelines selected included preventive measures, such as the sterilization of surgical material, operating room biosafety, or preoperative hand hygiene. Given that the effectiveness of these preventive measures has been widely demonstrated, they have not been included or described in the analysis. Finally, to analyze each of the CPG, a table was compiled to include the levels of evidence for each of the preventive measures, taking into account the following indications, adapted from the GRADE consensus (Grades of Recommendation, Assessment, Development, and Evaluation)8 (Table 1): “green”, defined as high-quality evidence to support the use of a measure; “orange”, defined as moderate-quality evidence to support the use of an accepted measure or practice; “white”, defined as insufficient evidence to support the use of said measure or that the state of the question is not yet fully resolved to be able to give a recommendation; or “red”, defined as high-quality evidence that does not support the use of a preventive measure, because it has been proven that it is not necessary for SSI prevention, or may even increase the risk for SSI.
Summary of Clinical Practice Guideline According to the Results of the Study's Search Criteria.
| Country, yr | Name | Institution or scientific society | Acronyms | Website | Methodology | Description of the methodology |
|---|---|---|---|---|---|---|
| United States, 2014 | Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update | The Society for Healthcare Epidemiology of America, the Infectious Disease Society of America, the Association for Professionals in Infection Control and Epidemiology, the American Hospital Association and the Joint Commission | SHEA, IDSA, APIC, AHA, JC | http://journals.cambridge.org/download.php?file=%2F2425_7E21D03310A55405100CA0AF6B672076_journals__ICE_ICE35_06_S0195941700093267a.pdf&cover=Y&code=5df3ee04574a699d14e1b68b258da7cd | Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) and the Canadian Task Force on Preventive Health Care | I. High-quality evidence: wide range of studies that do not have important limitations, little variation among the studies, and the estimated confidence interval is narrow II. Moderate-quality evidence: few studies and some with limitations; no important errors and some variation among the studies, or the estimated confidence interval is wide III. Low-quality evidence: the studies have important defects with much variation between them; there are no rigorous studies, only the consensus of experts. The estimated confidence interval is very wide. |
| United States, 2017 | The Guideline for the Prevention of Surgical Site Infection | The Healthcare Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention | HICPAC. (CDC) | http://jamanetwork.com/journals/jamasurgery/fullarticle/2623725?utm_campaign=articlePDF&utm_medium=articlePDFlink&utm_source=articlePDF&utm_content=jamasurg.2017.0904 | Recommendation grades to classify quality of evidence according to CDC and HICPAC | Category IA: strong recommendation supported by high or moderate-quality evidence. Category IB: strong recommendation supported by low-quality evidence. Category IC: strong recommendation required by state or federal regulation. Category II: weak recommendation supported by evidence of any level of quality. No recommendation/unresolved problem: an unresolved question for which there is low to very low quality evidence with uncertain discrepancies between the benefits and damages or no evidence published about the results considered critical to weigh the risks and benefits of an intervention |
| United Kingdom, England. 2008 and 2013 | Evidence Update June 2013. A summary of selected new evidence relevant to NICE clinical guideline 74 ‘Prevention and treatment of surgical site infection’ (2008) | The National Institute of Health and Clinical Excellence | NICE | http://www.nice.org.uk/guidance/cg74/evidence/evidence-update-241969645 | The clinical practice guidelines recommend/do not recommend based on the evidence found about the preventive measures. | 1++: high-quality meta-analyses, systematic reviews of clinical trials (RCT) or RCT with a very low risk for bias. 1+: well-developed meta-analyses, systematic reviews of RCT, or RCT with low risk for bias. 1−: meta-analyses, systematic reviews of RCT or RCT with a high risk for bias. 2++: high-quality systematic review of case or cohort studies; high-quality case-control studies or cohorts with a very low risk of confusion or bias and a high probability that the relationship is causal. 2+: well-developed studies of cases and controls or cohorts with a low risk of confusion or bias and a moderate probability that the relationship is causal. 2−: case–control or cohort studies with high risk of confusion, bias and a significant risk that the relationship is not causal. 3: non-analytical studies (for example, case reports, case series). 4: opinion of experts, formal consensus |
| Canada, 2014 | Safer Healthcare Now! Surgical Site Infection (SSI): Getting Started Kit | The Canadian Patient Safety Institute | CPSI | http://www.patientsafetyinstitute.ca/en/toolsresources/pages/SSI-resources-getting-started-kit.aspx | The clinical practice guidelines recommend/do not recommend, but with no levels of evidence. | Recommended or not recommended |
| United Kingdom, Scotland 2015 | Targeted literature review: What are the key infection prevention and control recommendations to inform a surgical site infection (SSI) prevention quality improvement tool? | The National Health Service Scotland | NHSS | http://www.documents.hps.scot.nhs.uk/hai/infection-control/evidence-for-care-bundles/literature-reviews/SSI-review-2015-02.pdf | Recommendation grades to define the quality of evidence according to CDC and HICPAC | Category IA: strong recommendation supported by high or moderate-quality evidence. Category IB: strong recommendation supported by low-quality evidence. Category IC: strong recommendation required by state or federal regulation. Category II: weak recommendation supported by evidence of any level of quality. No recommendation/unresolved problem: an unresolved question for which there is low to very low quality evidence with uncertain discrepancies between the benefits and damages or no evidence published about the results considered critical to weigh the risks and benefits of an intervention |
| Spain, 2010 | Clinical Practice Guidelines for the Surgical Patient Safety | National Healthcare System of the Spanish Ministry of Health, Social Policy and Equality | MSSSI | http://portal.guiasalud.es/eCPG/seguridad_paciente/completa/index.html | Guidelines based on GRADE | Strong: the beneficial effects of an intervention surpass the damages or strong opposing recommendation, in contrast, the damage of an intervention surpasses the beneficial effects. Weak or opposing weak: no conclusive evidence about the effects of an intervention. The guidelines provide a type of recommendation for those cases in which, in spite of there being no conclusive scientific evidence, identified with a “√”, the action is considered good clinical practice. |
| Switzerland, 2015 | Global Guidelines for the Prevention of Surgical Site Infection | World Health Organization | WHO | http://www.who.int/gpsc/ssi-prevention-guidelines/en/ | Guidelines based on GRADE | High: the true effect is considered closed to the estimated effect. Moderate: the true effect is probably close to the estimated effect, but it is possible that it is substantially different. Low: the true effect may be substantially different from the estimated effect. Very low: the true effect is most likely substantially different from the estimated effect. |
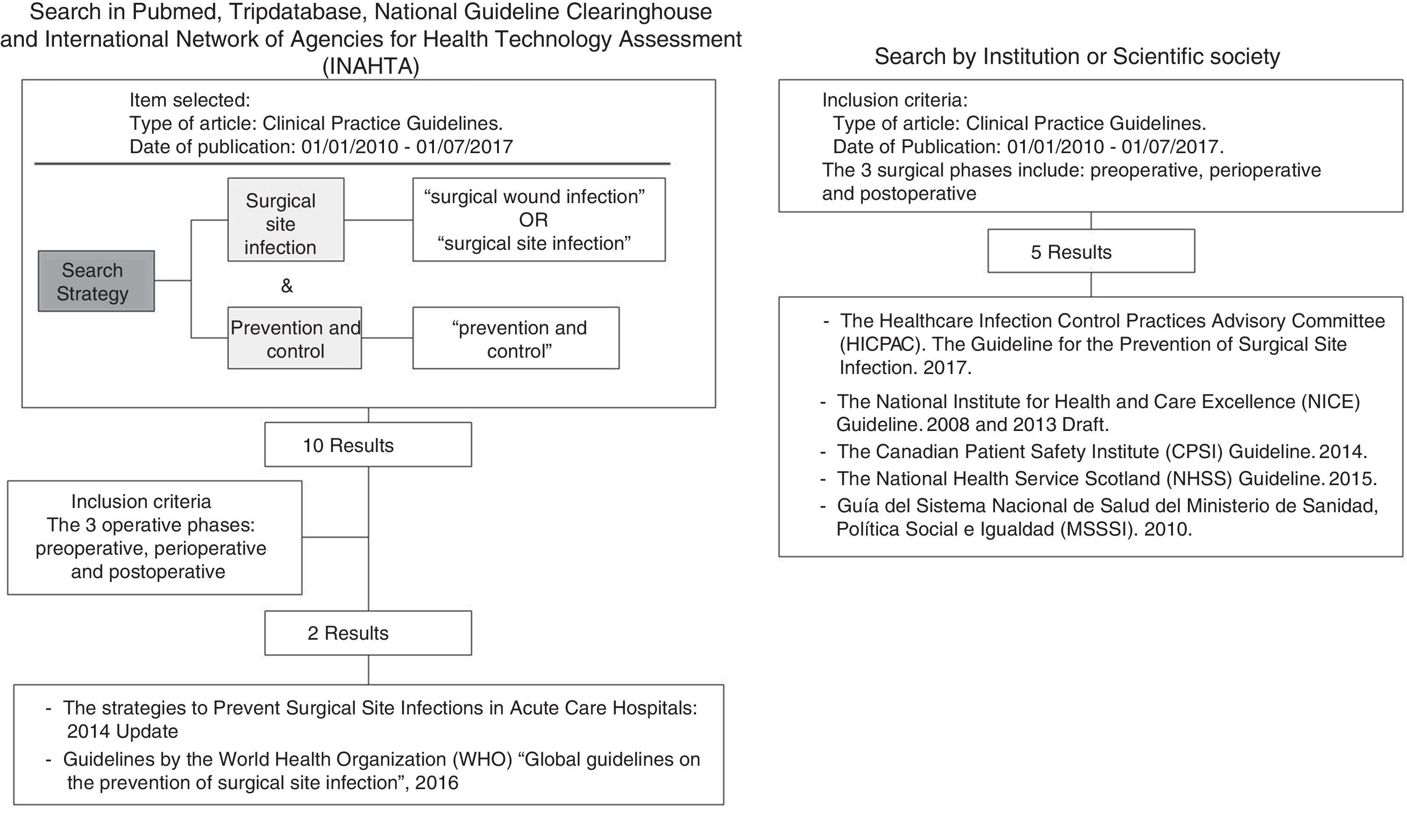
The search uncovered 15 bibliographic references. There were no duplicate references, so the selection of articles was determined according to the inclusion criteria set forth above. Fig. 1 shows the search strategy and flowchart for document management. In the end, 7 documents were selected for analysis:
- 1.
The strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update Guideline8 (SHEA)
- 2.
The Healthcare Infection Control Practices Advisory Committee (HICPAC), Centers for Disease Control and Prevention. The Guideline for the Prevention of Surgical Site Infection, 201721
- 3.
The National Institute of Health and Clinical Excellence (NICE) Guideline, published in 2008,22 and the Draft Guideline from 201323,24
- 4.
The Canadian Patient Safety Institute Guideline (CPSI), 201425
- 5.
The National Health Service Scotland Guideline (NHSS), 201526
- 6.
Clinical Practical Guidelines for the Safety of Surgical Patients. The 2010 guidelines of the National Healthcare System of the Spanish Ministry of Health, Social Services and Equality (MSSSI)5
- 7.
World Health Organizaton (WHO) guidelines, “Global guidelines on the prevention of surgical site infection”, from 201627
Table 1 includes the main characteristics to be noted in each of the selected CPG: country, year of publication, specific title of the guidelines, institution or scientific society that has developed the guidelines and any acronyms, a link to the web page, evaluation scale of the evidence used and a detailed description of the criteria of that scale. We noted that there was no unanimity in the assessment scales used. The MSSSI and SHEA guidelines used the GRADE system, the HICPAC, NHSS and NICE guidelines used their own classifications, and the CPSI used a simpler classification (recommended/not recommended).
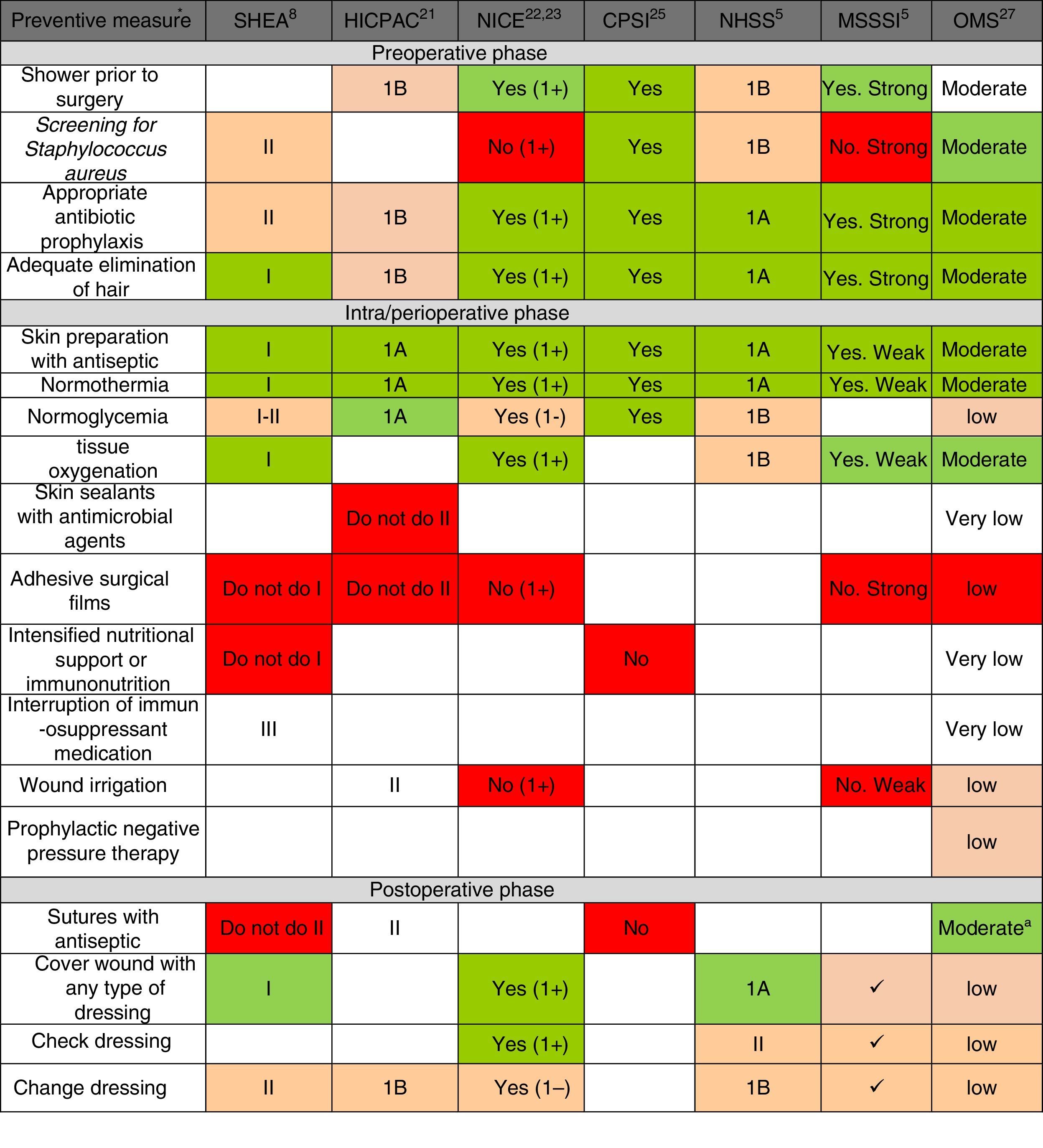
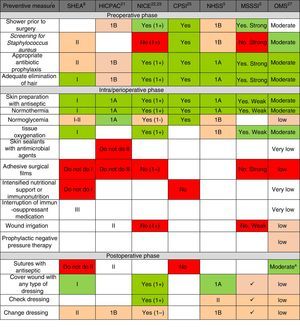
Fig. 2 shows the main preventive measures, divided into the 3 healthcare phases (preoperative, perioperative and postoperative) with the assessment of the quality of evidence and recommendation grade given by each of the selected CPG. In general, there is a greater quality of the recommendations in the pre- and perioperative phases, while the recommendations are lower grade for postoperative preventive measures. Only the WHO CPG made recommendations for all points, while the HICPAC and CPSI omit various recommendations for preventive measures in the perioperative and postoperative phases.
Summary of recommendation grades and quality of evidence, according to the review of the preventive measures, in accordance with clinical guidelines. CPSI: Canadian Patient Safety Institute; HICPAC: Healthcare Infection Control Practices Advisory Committee. Center for Disease Control and Prevention; MSSSI: Spanish Ministry for Healthcare, Social Policy and Equality; NHSS: National Health Service Scotland; NICE: National Institute of Health and Clinical Excellence; WHO: World Health Organization; SHEA: Society for Healthcare Epidemiology of America. a Considering the low-moderate quality of the evidence and the comparisons in the subgroups of the randomized clinical trials included in the analysis of the meta-regression, the WHO agree that the strength of the recommendation should be conditional. * The recommendation grades and quality of the evidence are described in the methodology and in Table 1. The recommendation of the measure is marked by the color scale: “Yes, Recommended” is green, defined by high-quality evidence supporting the use of a measure; orange represents moderate-quality evidence supporting the use of a measure or accepted practice; white represents insufficient evidence to support (or not) the use of that measure, or the state of the question has not yet been fully resolved to be able to give a recommendation; and “Not recommended” is red, defined by high-quality evidence that does not support the use of a preventive measure, because it has been shown that it is not necessary for SSI prevention or may even increase the risk for SSI.
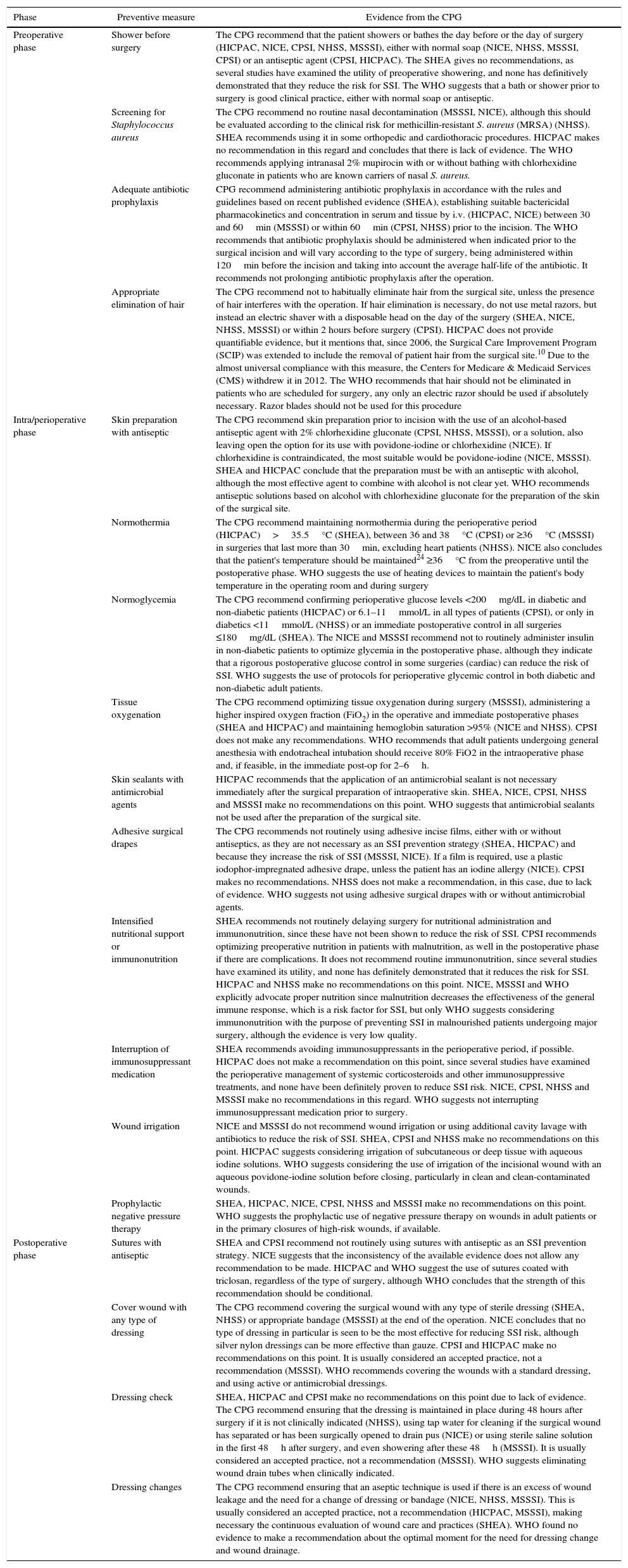
Regarding each of the generic preventive recommendations, Table 2 illustrates the key points that support these recommendations for each CPG. Many disagreements between CPG appeared in aspects such as showering before surgery, Staphylococcus aureus screening, application of normothermia (minimum temperature to be maintained) and normoglycemia (glucose levels in diabetics and non-diabetics), among others.
Description of Preventive Measures According to Clinical Practice.
| Phase | Preventive measure | Evidence from the CPG |
|---|---|---|
| Preoperative phase | Shower before surgery | The CPG recommend that the patient showers or bathes the day before or the day of surgery (HICPAC, NICE, CPSI, NHSS, MSSSI), either with normal soap (NICE, NHSS, MSSSI, CPSI) or an antiseptic agent (CPSI, HICPAC). The SHEA gives no recommendations, as several studies have examined the utility of preoperative showering, and none has definitively demonstrated that they reduce the risk for SSI. The WHO suggests that a bath or shower prior to surgery is good clinical practice, either with normal soap or antiseptic. |
| Screening for Staphylococcus aureus | The CPG recommend no routine nasal decontamination (MSSSI, NICE), although this should be evaluated according to the clinical risk for methicillin-resistant S. aureus (MRSA) (NHSS). SHEA recommends using it in some orthopedic and cardiothoracic procedures. HICPAC makes no recommendation in this regard and concludes that there is lack of evidence. The WHO recommends applying intranasal 2% mupirocin with or without bathing with chlorhexidine gluconate in patients who are known carriers of nasal S. aureus. | |
| Adequate antibiotic prophylaxis | CPG recommend administering antibiotic prophylaxis in accordance with the rules and guidelines based on recent published evidence (SHEA), establishing suitable bactericidal pharmacokinetics and concentration in serum and tissue by i.v. (HICPAC, NICE) between 30 and 60min (MSSSI) or within 60min (CPSI, NHSS) prior to the incision. The WHO recommends that antibiotic prophylaxis should be administered when indicated prior to the surgical incision and will vary according to the type of surgery, being administered within 120min before the incision and taking into account the average half-life of the antibiotic. It recommends not prolonging antibiotic prophylaxis after the operation. | |
| Appropriate elimination of hair | The CPG recommend not to habitually eliminate hair from the surgical site, unless the presence of hair interferes with the operation. If hair elimination is necessary, do not use metal razors, but instead an electric shaver with a disposable head on the day of the surgery (SHEA, NICE, NHSS, MSSSI) or within 2 hours before surgery (CPSI). HICPAC does not provide quantifiable evidence, but it mentions that, since 2006, the Surgical Care Improvement Program (SCIP) was extended to include the removal of patient hair from the surgical site.10 Due to the almost universal compliance with this measure, the Centers for Medicare & Medicaid Services (CMS) withdrew it in 2012. The WHO recommends that hair should not be eliminated in patients who are scheduled for surgery, any only an electric razor should be used if absolutely necessary. Razor blades should not be used for this procedure | |
| Intra/perioperative phase | Skin preparation with antiseptic | The CPG recommend skin preparation prior to incision with the use of an alcohol-based antiseptic agent with 2% chlorhexidine gluconate (CPSI, NHSS, MSSSI), or a solution, also leaving open the option for its use with povidone-iodine or chlorhexidine (NICE). If chlorhexidine is contraindicated, the most suitable would be povidone-iodine (NICE, MSSSI). SHEA and HICPAC conclude that the preparation must be with an antiseptic with alcohol, although the most effective agent to combine with alcohol is not clear yet. WHO recommends antiseptic solutions based on alcohol with chlorhexidine gluconate for the preparation of the skin of the surgical site. |
| Normothermia | The CPG recommend maintaining normothermia during the perioperative period (HICPAC)>35.5°C (SHEA), between 36 and 38°C (CPSI) or ≥36°C (MSSSI) in surgeries that last more than 30min, excluding heart patients (NHSS). NICE also concludes that the patient's temperature should be maintained24 ≥36°C from the preoperative until the postoperative phase. WHO suggests the use of heating devices to maintain the patient's body temperature in the operating room and during surgery | |
| Normoglycemia | The CPG recommend confirming perioperative glucose levels <200mg/dL in diabetic and non-diabetic patients (HICPAC) or 6.1–11mmol/L in all types of patients (CPSI), or only in diabetics <11mmol/L (NHSS) or an immediate postoperative control in all surgeries ≤180mg/dL (SHEA). The NICE and MSSSI recommend not to routinely administer insulin in non-diabetic patients to optimize glycemia in the postoperative phase, although they indicate that a rigorous postoperative glucose control in some surgeries (cardiac) can reduce the risk of SSI. WHO suggests the use of protocols for perioperative glycemic control in both diabetic and non-diabetic adult patients. | |
| Tissue oxygenation | The CPG recommend optimizing tissue oxygenation during surgery (MSSSI), administering a higher inspired oxygen fraction (FiO2) in the operative and immediate postoperative phases (SHEA and HICPAC) and maintaining hemoglobin saturation >95% (NICE and NHSS). CPSI does not make any recommendations. WHO recommends that adult patients undergoing general anesthesia with endotracheal intubation should receive 80% FiO2 in the intraoperative phase and, if feasible, in the immediate post-op for 2–6h. | |
| Skin sealants with antimicrobial agents | HICPAC recommends that the application of an antimicrobial sealant is not necessary immediately after the surgical preparation of intraoperative skin. SHEA, NICE, CPSI, NHSS and MSSSI make no recommendations on this point. WHO suggests that antimicrobial sealants not be used after the preparation of the surgical site. | |
| Adhesive surgical drapes | The CPG recommends not routinely using adhesive incise films, either with or without antiseptics, as they are not necessary as an SSI prevention strategy (SHEA, HICPAC) and because they increase the risk of SSI (MSSSI, NICE). If a film is required, use a plastic iodophor-impregnated adhesive drape, unless the patient has an iodine allergy (NICE). CPSI makes no recommendations. NHSS does not make a recommendation, in this case, due to lack of evidence. WHO suggests not using adhesive surgical drapes with or without antimicrobial agents. | |
| Intensified nutritional support or immunonutrition | SHEA recommends not routinely delaying surgery for nutritional administration and immunonutrition, since these have not been shown to reduce the risk of SSI. CPSI recommends optimizing preoperative nutrition in patients with malnutrition, as well in the postoperative phase if there are complications. It does not recommend routine immunonutrition, since several studies have examined its utility, and none has definitely demonstrated that it reduces the risk for SSI. HICPAC and NHSS make no recommendations on this point. NICE, MSSSI and WHO explicitly advocate proper nutrition since malnutrition decreases the effectiveness of the general immune response, which is a risk factor for SSI, but only WHO suggests considering immunonutrition with the purpose of preventing SSI in malnourished patients undergoing major surgery, although the evidence is very low quality. | |
| Interruption of immunosuppressant medication | SHEA recommends avoiding immunosuppressants in the perioperative period, if possible. HICPAC does not make a recommendation on this point, since several studies have examined the perioperative management of systemic corticosteroids and other immunosuppressive treatments, and none have been definitely proven to reduce SSI risk. NICE, CPSI, NHSS and MSSSI make no recommendations in this regard. WHO suggests not interrupting immunosuppressant medication prior to surgery. | |
| Wound irrigation | NICE and MSSSI do not recommend wound irrigation or using additional cavity lavage with antibiotics to reduce the risk of SSI. SHEA, CPSI and NHSS make no recommendations on this point. HICPAC suggests considering irrigation of subcutaneous or deep tissue with aqueous iodine solutions. WHO suggests considering the use of irrigation of the incisional wound with an aqueous povidone-iodine solution before closing, particularly in clean and clean-contaminated wounds. | |
| Prophylactic negative pressure therapy | SHEA, HICPAC, NICE, CPSI, NHSS and MSSSI make no recommendations on this point. WHO suggests the prophylactic use of negative pressure therapy on wounds in adult patients or in the primary closures of high-risk wounds, if available. | |
| Postoperative phase | Sutures with antiseptic | SHEA and CPSI recommend not routinely using sutures with antiseptic as an SSI prevention strategy. NICE suggests that the inconsistency of the available evidence does not allow any recommendation to be made. HICPAC and WHO suggest the use of sutures coated with triclosan, regardless of the type of surgery, although WHO concludes that the strength of this recommendation should be conditional. |
| Cover wound with any type of dressing | The CPG recommend covering the surgical wound with any type of sterile dressing (SHEA, NHSS) or appropriate bandage (MSSSI) at the end of the operation. NICE concludes that no type of dressing in particular is seen to be the most effective for reducing SSI risk, although silver nylon dressings can be more effective than gauze. CPSI and HICPAC make no recommendations on this point. It is usually considered an accepted practice, not a recommendation (MSSSI). WHO recommends covering the wounds with a standard dressing, and using active or antimicrobial dressings. | |
| Dressing check | SHEA, HICPAC and CPSI make no recommendations on this point due to lack of evidence. The CPG recommend ensuring that the dressing is maintained in place during 48 hours after surgery if it is not clinically indicated (NHSS), using tap water for cleaning if the surgical wound has separated or has been surgically opened to drain pus (NICE) or using sterile saline solution in the first 48h after surgery, and even showering after these 48h (MSSSI). It is usually considered an accepted practice, not a recommendation (MSSSI). WHO suggests eliminating wound drain tubes when clinically indicated. | |
| Dressing changes | The CPG recommend ensuring that an aseptic technique is used if there is an excess of wound leakage and the need for a change of dressing or bandage (NICE, NHSS, MSSSI). This is usually considered an accepted practice, not a recommendation (HICPAC, MSSSI), making necessary the continuous evaluation of wound care and practices (SHEA). WHO found no evidence to make a recommendation about the optimal moment for the need for dressing change and wound drainage. |
CPSI: Canadian Patient Safety Institute; CPG: clinical practice guidelines; HICPAC: Healthcare Infection Control Practices Advisory Committee. Center for Disease Control and Prevention; SSI: surgical site infection; MSSSI: Spanish Ministry of Healthcare, Social Policy and Equality; NHSS: National Health Service Scotland; NICE: National Institute of Health and Clinical Excellence; WHO: World Health Organization; SHEA: Society for Healthcare Epidemiology of America.
We believe that the results obtained meet the defined objective of making a current synthesis of CPG recommendations for the prevention of SSI. Seven CPG were selected, written by institutions and official bodies that have assessed the best scientific evidence available to prevent SSI in the 3 phases of surgical treatment. There was lower evidence detected in the postoperative phase, which is consistent with current SSI etiological models.2
Following current CPG quality recommendations, most CPG provide a double assessment on the level of evidence and the level of recommendation, as in the GRADE system.5,8,21,26,27 However, some use their own recommendation systems, which makes comparisons difficult.23,25 The formal drafting of some recommendations of measures (for instance “without sufficient evidence to be able to support the use of this recommendation” “unresolved issue”) are also no help when trying to equate the recommendations among the CPG. There is a certain disparity between the level of evidence and the recommendation grade, as in the case of normothermia, where some CPG recommend maintaining very demanding temperature levels between 36°C and 38°C (CPSI) and other indications are more lax at >35.5°C (SHEA). Likewise, it is not clear if the application of these measures must be carried out in the perioperative phase or only in the intraoperative period. With glycemia, the situation is similar, since it is not clear if the maximum level of glycemia allowed (180 vs 200mg/dL) should be applied only to diabetic patients, if it should be met in the 3 phases and if it would be indicated according to the type of intervention.
Furthermore, when comparing different CPG, we observed that only 4 measures were recommended by all guidelines and institutions: appropriate elimination of hair, antibiotic prophylaxis, preparation of the surgical field with an alcohol-based product (most CPG recommend alcoholic chlorhexidine [AC]) and normothermia. Other measures, however, such as the screening of S. aureus and preoperative showering with soap, are recommended in few CPG.5,8,21–23,25–27 In addition, the majority coincided in not recommending measures such as plastic incise drapes5,8,21–23 or antimicrobial agents in sutures.8,25 Specifically, the latter is recommended in only one set of guidelines, although with very limited evidence,27 whereas screening for S. aureus, for example, remains a controversial practice. Some guidelines recommend screening for methicillin-resistant S. aureus (MRSA),26 while others simply recommend screening for S. aureus,25 and others do not even recommend screening due to lack of evidence.21
Although there is unanimity in recommending the 4 mentioned preventive measures, slight variations are given in the specific recommendations and in the critical points that specify these recommendations, such as dosages of the antibiotic as prophylaxis or administration prior to the incision. The same occurs with the type of antiseptic applicator for the preparation of the skin, whereas other key issues are not discussed, such as the friction technique or the number of times the product should be applied. While to date no systematic reviews have been published on the effectiveness of normothermia and normoglycemia, the CPG coincide in recommending them. In the case of normothermia control recommendations, the optimal monitoring strategy for central temperature is not specified,5,8,21,23,27 although the guidelines are clear in recommending its use21,24,25,27 and are equally clear about the control of glycemia and diabetes.28
Certain habits are deeply rooted in the operating room, which might explain the vagueness of the CPG regarding skin antisepsis. The change from povidone-iodine to tinted 2% AC is an important change of habits in the operating theater.29 The use of an alcohol-based product may also pose a certain risk of ignition if it is not applied safely. In the USA, there was an estimated frequency of 50–200 burn episodes out of more than 51 million interventions in 2010, mostly associated with the use of AC.30 The use of alcohol solutions has recently been supported as a safe and effective measure for the prevention of SSI in 2 Cochrane reviews in 201331 and 2015.32 Furthermore, there are proven measures to avoid intraoperative ignition, such as the use of applicators that dissipate and control AC vapors, careful revision of the surgical field in search of spills, and the controlled dosage of antiseptic according to the amount expected for each intervention, which can be efficiently adjusted to the product to be used.13,15
In the last decade, some Cochrane reviews have arisen with preoperative measures that provide for better estimations of the effectiveness of antibiotic prophylaxis in certain types of surgeries, such as the colon,20 and specific administration times, such as before clamping the umbilical cord in cesarean sections.33,34 Likewise, it has been demonstrated that strictly evaluating compliance of key aspects of antibiotic prophylaxis,14,35 such as administration within 60min prior to surgery, the proportion of compliance in 2 studies went from 40%36 and 68%,37 to 91% and 99%, respectively. Other main measures studied in systematic reviews and consequently recommended have been the elimination of hair20 or perioperative tissue oxygenation, although some recent reviews suggest that a fraction of inspired oxygen of 60% or more still lacks solid evidence to be able to be recommended systematically.38
New preventive measures have been suggested by some studies, but for the moment they have not merited explicit recommendation in the CPG. This is the case of the use of film incise drapes, with or without antimicrobial agents, which the guides suggest not to use due to the lack of evidence for SSI prevention.5,8,23 Some authors even argue against their use, due to an observed increase in infection rates.39,40 The same is true with antimicrobial-coated sutures, which seem to reduce the rates of infection in some types of surgery,21,23 but not at all.8,25
The type of wound dressing after surgery is a subject that is in full discussion. There is not sufficient evidence about the benefits of covering surgical wounds that heal by primary intention with dressings, and there is no type of dressing in particular that is more effective in reducing SSI rates, improving healing, controlling pain, acceptable by the patient or as easy to remove.41,42 The other 2 measures reviewed in the postoperative phase (dressing check/change) have had no quality studies demonstrating their effectiveness to reduce SSI.26 However, it should be noted that the early withdrawal of the dressing can significantly reduce hospital stay and costs, compared with covering the wound with dressings for more than 48hours after surgery.41
The systematic review and updating of the most effective measures for the prevention of SSI should soon be translated into effective implementation of SSI prevention programs in our hospitals, since it is a priority to implement the most effective preventative measures above other guidelines that are less supported by scientific evidence.43 There is still a significant deficit in the application of the “bundles” or sets of SSI prevention measures, which have been proven to be fully feasible and based on the most effective preventative measures. On the other hand, although in this document we have evaluated the utility of supposedly general preventative measures, we must realize that these are not applicable in all surgeries or all patients, so each center must define its indications of use according to the types of intervention, define the critical points that ensure their application, and choose the bundles that best suit their resources. It has been found that, despite good adherence, the preventive effectiveness of guidelines sets is very variable in different settings.44,45 A key aspect for the preventive measures to be effective is, therefore, verification of their correct compliance and the incorporation of the measures into the culture of quality care. Surgical checklists are a good tool to introduce new measures, and they are widely accepted in the healthcare environment,46 although they are not exempt from errors due to poor compliance.47 In practice, their implementation has been uneven and has often come up against a deficient safety culture in hospitals.15,16,48–50
In conclusion, patient safety projects have shown that a change in the culture of patient-oriented safety is required.48 Given that there is sufficient evidence to recommend a set of common SSI prevention measures, and that these can be easily organized in bundles or sets, various specific projects for HAI prevention have been appearing throughout the world, and also in our country.51,52 Specifically, there is the nationwide Zero Surgical Infection Project (2014),52 supported by the MSSSI, autonomous communities and the main scientific societies involved. This initiative includes 5 preventive measures that are clearly supported by scientific evidence, 3 of which are mandatory (antibiotic prophylaxis, skin antisepsis and no hair elimination) and 2 are optional (normothermia and normoglycemia), which coincides with the most recommended measures of the CPG examined. Thus, this project pays special attention to the compliance with critical points that define each preventive intervention and the verification of their compliance by means of checklists.
Conflict of InterestsNone.
Please cite this article as: Gómez-Romero FJ, Fernández-Prada M, Navarro-Gracia JF. Prevención de la infección de sitio quirúrgico: análisis y revisión narrativa de las guías de práctica clínica. Cir Esp. 2017;95:490–502.