Sentinel lymph node biopsy and ACOSOG-Z0011 criteria have modified axillary treatment in breast cancer surgery. We performed a systematic review of studies assessing the impact of axillary treatment on survival. The search showed 6891 potentially eligible items. Of them, 23 clinical trials and 12 meta-analyses published between 1980 and 2017 met the study criteria. The review revealed that axillary lymph node dissection (ALND) can be omitted in patients pN0 and pN1mic, without compromising survival. In patients pN1 it is proposed not to treat the axilla or replace ALND for axillary radiotherapy. The main limitations of this study are the inclusion of old tests that do not use therapeutic targets and lack of risk categorization of relapse. In conclusion, axillary treatment can be avoided in patients without metastatic involvement or micrometastases in the sentinel lymph node. However, there is no evidence to make a recommendation of axillary treatment in N1 patients, so individualized analysis of patient risk factors is needed.
La biopsia de ganglio centinela (BGC) y los criterios ACOSOG-Z0011 han modificado el tratamiento axilar en la cirugía primaria del cáncer de mama. Por esto se realiza una revisión sistemática de los estudios que valoran el impacto del tratamiento axilar en la supervivencia. La búsqueda mostró 6.891 artículos potencialmente elegibles, de los cuales, 23 ensayos clínicos y 12 metaanálisis publicados entre 1980 y 2017 cumplieron los criterios del estudio. La revisión desveló que la linfadenectomía axilar (LA) puede ser omitida en pacientes pN0 y pN1mic, sin comprometer la supervivencia. En pacientes pN1, se propone no tratar la axila o sustituir la LA por radioterapia axilar (RA). Las principales limitaciones de este estudio es que los ensayos son antiguos, no utilizan terapias dianas ni categorizan el riesgo de recaída. En conclusión, el tratamiento axilar puede ser suprimido en pacientes sin afectación metastásica o con micrometástasis del ganglio centinela. No obstante, no hay evidencia para establecer una recomendación de tratamiento axilar en las pacientes con afectación ganglionar N1, por lo que precisan de un análisis individualizado de sus factores de riesgo.
In recent years, we have witnessed a change in decision-making for adjuvant treatment in women with breast cancer. Previously, disease staging based on tumor size and lymph node involvement were the elements that determined the choice of locoregional and systemic treatment. Currently, biological factors of the tumor are the basis for the selection of systemic treatment, and the choice of drugs is almost exclusively defined by the immunohistochemical or genetic characteristics of the tumor.1–4 On the other hand, the decision of axillary treatment has not experienced this evolution and, consequently, axillary staging continues to be the key factor for the indication of axillary lymph node dissection (ALND) or axillary radiotherapy (ART). At present, this decision is controversial, for different reasons. The first, old clinical trials (CT)5–10 with selected groups of patients have indicated that axillary treatment does not have an impact on overall survival (OS). Secondly, other trials11–13 show that ALND can be suppressed in a select group of women with micrometastatic involvement of the sentinel lymph node (SLN), without compromising disease-free survival (DFS) or OS. Finally, some CT14,15 have demonstrated the non-inferiority of ART versus ALND in women with metastatic SLN, with a lower incidence of lymphedema. These facts have resulted in modifications in axillary treatment strategy in women with N1 (1–3 lymph nodes) involvement.
The objective of this study is to develop a systematic review in order to analyze the impact of axillary treatment (ALND, ART) in primary surgery for breast cancer, with the aim to establish clinical recommendations.
MethodA bibliographic search was carried out in PubMed, the Cochrane Library and Academic Google with the search terms: “axillary lymph node dissection”, “axillary radiotherapy” and “micrometastasis”, in association with the words “breast cancer”. The search was formulated according to the PICOS strategy where P was: women with breast cancer and primary surgery; I: axillary lymphadenectomy; C: ART or follow-up; O: OS and morbidity; S: clinical trials and meta-analyses. The PRISMA16 methodology was used. A search of clinical trials presently underway was also done on www.clinicaltrials.gov.
Inclusion and exclusion criteria: included in the study were CT that have analyzed OS with a mean follow-up of at least 5 years, as well as quality meta-analyses that comparing axillary treatments published between 1980 and 2017 in Spanish or English. The study population was comprised of women with primary surgery for their illness (Tis-T4a, N0–N3, M0). Finally, this review included CT that are currently underway and are analyzing the impact of axillary treatment on survival, with the aim to discuss lines of future research. Excluded from the study were duplicate studies, those published in other languages and those that, due to their methodology, follow-up time or number of patients included, were not considered relevant. Similarly excluded were those CT and meta-analyses that either did not report OS or included patients with neoadjuvant chemotherapy or metastatic breast cancer (stage IV).
Two reviewers (BA and AGN) examined the titles and abstracts of the references uncovered in the search to identify potentially eligible publications. The full text of the selected articles was obtained after reading the title/summary, and the selection criteria were applied to review each trial. The 2 reviewers independently evaluated each of the trials that were potentially eligible for inclusion in the review, and discrepancies were resolved by discussion. By mutual agreement, those of greatest importance were chosen. The selected CT and meta-analyses analyzed axillary treatment in primary breast cancer surgery. Finally, a critical analysis was carried out to establish recommendations for clinical practice. The level of evidence and recommendation grades were established according to criteria of the US Preventive Service Task Force.17
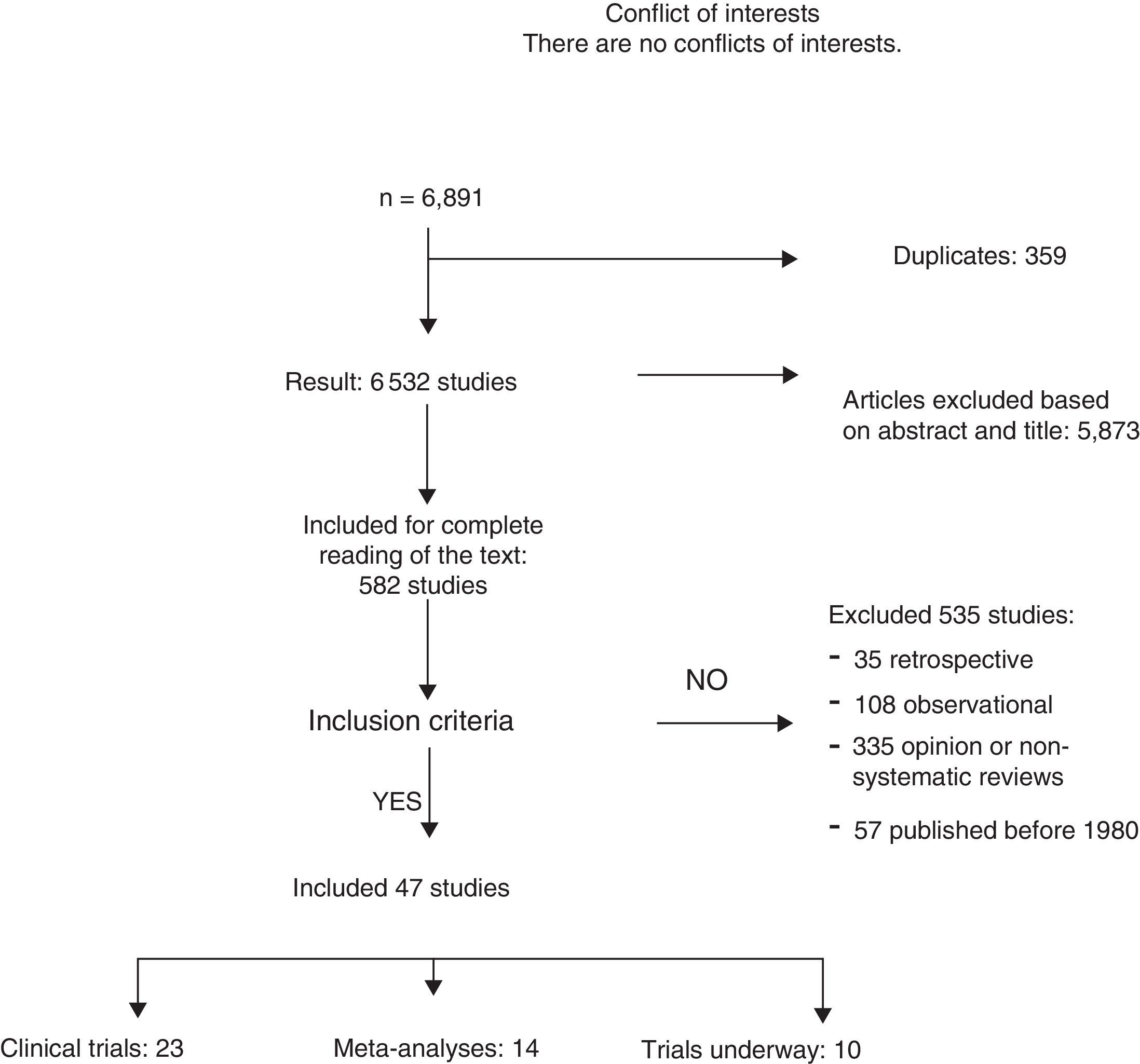
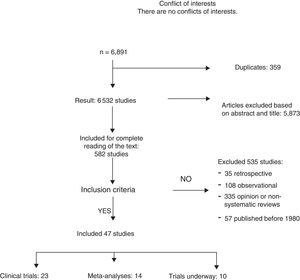
ResultsA total of 6891 articles were potentially eligible, and 359 of these were duplications. After reading the title or the abstract, we excluded 5873 studies as they were not related with the subject and 535 for the following reasons: 35 for being retrospective, 108 for being observational studies; 335 for opinion or with incorrect methodology; and 57 for being published before 1980. A total of 23 CT and 12 meta-analyses were included in the systematic review (Fig. 1). The search identified 13 CT in progress that studied axillary treatment in different clinical settings.
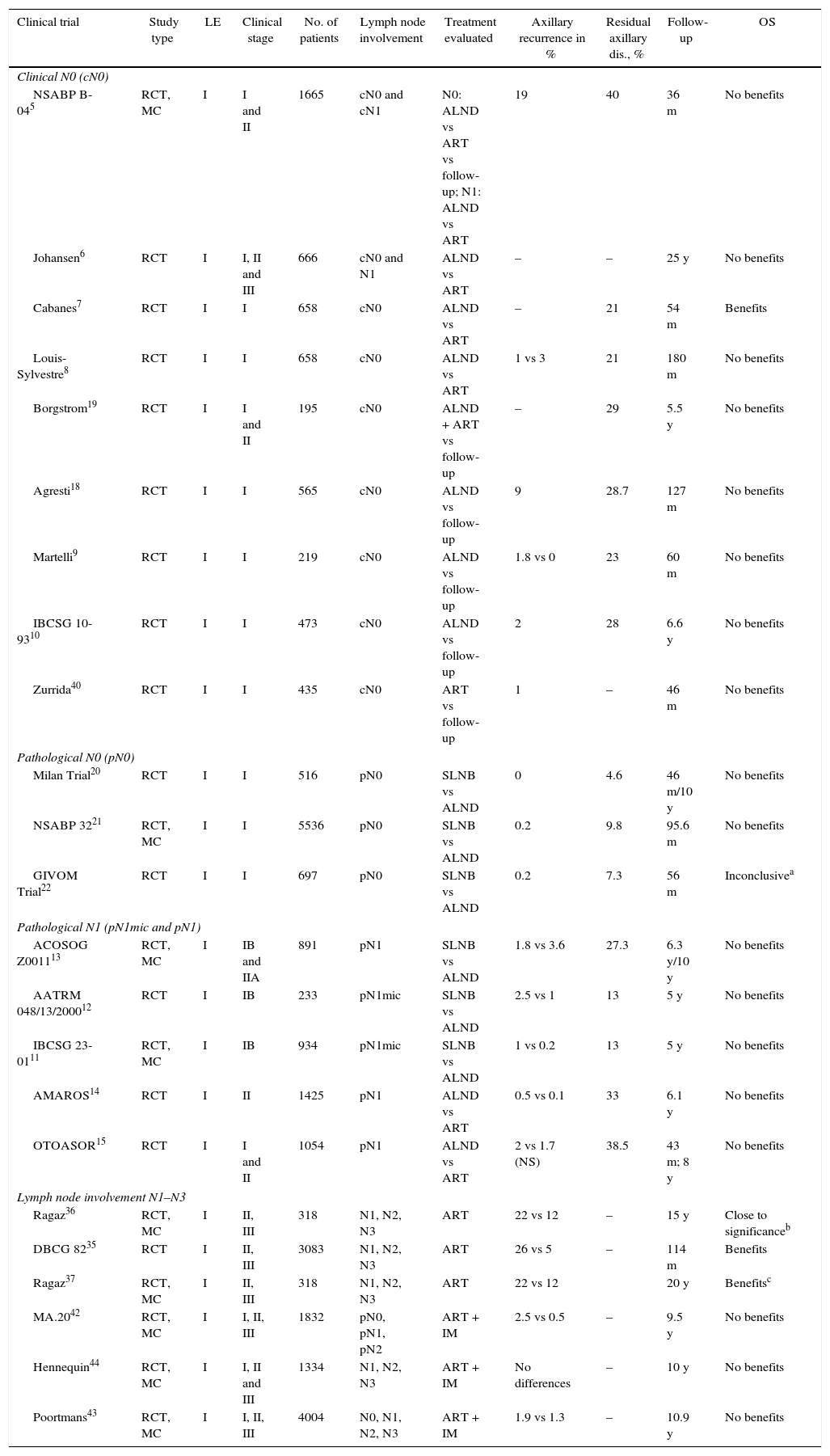
Axillary Lymph Node Dissection in Patients Without Lymph Node Involvement (N0)Our bibliographic search identified 7 CT that analyzed the impact of ALND in the OS of patients with no clinical axillary involvement (cN0) prior to the introduction of sentinel lymph node biopsy (SLNB).6,18,19 Four of them9,10,18,19 compared ALND with the follow-up and did not show significant differences in the OS of both groups after a mean follow-up of at least 5 years. However, 2 of these CT9,10 only included elderly patients. Another 2 CT compared ALND versus ART.6–8 One of them, by Cabanes et al.,7 reported a significant benefit of ALND; however, this benefit disappeared in the long-term follow-up (180 months). Finally, the NSABP B-04 study randomized patients with mastectomy for ALND, ART and follow-up, with no evidence of benefits in DFS or OS in patients with clinically negative axillary nodes (cN0) without ALND (Table 1).
Clinical Trials That Analyze the Impact of Axillary Treatment (ALND and ART) on Overall Survival.
| Clinical trial | Study type | LE | Clinical stage | No. of patients | Lymph node involvement | Treatment evaluated | Axillary recurrence in % | Residual axillary dis., % | Follow-up | OS |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical N0 (cN0) | ||||||||||
| NSABP B-045 | RCT, MC | I | I and II | 1665 | cN0 and cN1 | N0: ALND vs ART vs follow-up; N1: ALND vs ART | 19 | 40 | 36 m | No benefits |
| Johansen6 | RCT | I | I, II and III | 666 | cN0 and N1 | ALND vs ART | – | – | 25 y | No benefits |
| Cabanes7 | RCT | I | I | 658 | cN0 | ALND vs ART | – | 21 | 54 m | Benefits |
| Louis-Sylvestre8 | RCT | I | I | 658 | cN0 | ALND vs ART | 1 vs 3 | 21 | 180 m | No benefits |
| Borgstrom19 | RCT | I | I and II | 195 | cN0 | ALND + ART vs follow-up | – | 29 | 5.5 y | No benefits |
| Agresti18 | RCT | I | I | 565 | cN0 | ALND vs follow-up | 9 | 28.7 | 127 m | No benefits |
| Martelli9 | RCT | I | I | 219 | cN0 | ALND vs follow-up | 1.8 vs 0 | 23 | 60 m | No benefits |
| IBCSG 10-9310 | RCT | I | I | 473 | cN0 | ALND vs follow-up | 2 | 28 | 6.6 y | No benefits |
| Zurrida40 | RCT | I | I | 435 | cN0 | ART vs follow-up | 1 | – | 46 m | No benefits |
| Pathological N0 (pN0) | ||||||||||
| Milan Trial20 | RCT | I | I | 516 | pN0 | SLNB vs ALND | 0 | 4.6 | 46 m/10 y | No benefits |
| NSABP 3221 | RCT, MC | I | I | 5536 | pN0 | SLNB vs ALND | 0.2 | 9.8 | 95.6 m | No benefits |
| GIVOM Trial22 | RCT | I | I | 697 | pN0 | SLNB vs ALND | 0.2 | 7.3 | 56 m | Inconclusivea |
| Pathological N1 (pN1mic and pN1) | ||||||||||
| ACOSOG Z001113 | RCT, MC | I | IB and IIA | 891 | pN1 | SLNB vs ALND | 1.8 vs 3.6 | 27.3 | 6.3 y/10 y | No benefits |
| AATRM 048/13/200012 | RCT | I | IB | 233 | pN1mic | SLNB vs ALND | 2.5 vs 1 | 13 | 5 y | No benefits |
| IBCSG 23-0111 | RCT, MC | I | IB | 934 | pN1mic | SLNB vs ALND | 1 vs 0.2 | 13 | 5 y | No benefits |
| AMAROS14 | RCT | I | II | 1425 | pN1 | ALND vs ART | 0.5 vs 0.1 | 33 | 6.1 y | No benefits |
| OTOASOR15 | RCT | I | I and II | 1054 | pN1 | ALND vs ART | 2 vs 1.7 (NS) | 38.5 | 43 m; 8 y | No benefits |
| Lymph node involvement N1–N3 | ||||||||||
| Ragaz36 | RCT, MC | I | II, III | 318 | N1, N2, N3 | ART | 22 vs 12 | – | 15 y | Close to significanceb |
| DBCG 8235 | RCT | I | II, III | 3083 | N1, N2, N3 | ART | 26 vs 5 | – | 114 m | Benefits |
| Ragaz37 | RCT, MC | I | II, III | 318 | N1, N2, N3 | ART | 22 vs 12 | 20 y | Benefitsc | |
| MA.2042 | RCT, MC | I | I, II, III | 1832 | pN0, pN1, pN2 | ART + IM | 2.5 vs 0.5 | – | 9.5 y | No benefits |
| Hennequin44 | RCT, MC | I | I, II and III | 1334 | N1, N2, N3 | ART + IM | No differences | – | 10 y | No benefits |
| Poortmans43 | RCT, MC | I | I, II, III | 4004 | N0, N1, N2, N3 | ART + IM | 1.9 vs 1.3 | – | 10.9 y | No benefits |
y: years; RCT: randomized clinical trial; FN: false negative; m: months; MC: multicenter; IM: internal mammary; LE: level of evidence; ART: axillary radiotherapy; OS: overall survival.
Three EC,20–22 that included more than 7000 patients, have compared SLNB to ALND in patients without pathological involvement of the axilla (pN0) and have showed a similar incidence of axillary recurrence and OS, with less morbidity in patients with SLNB (Table 1).
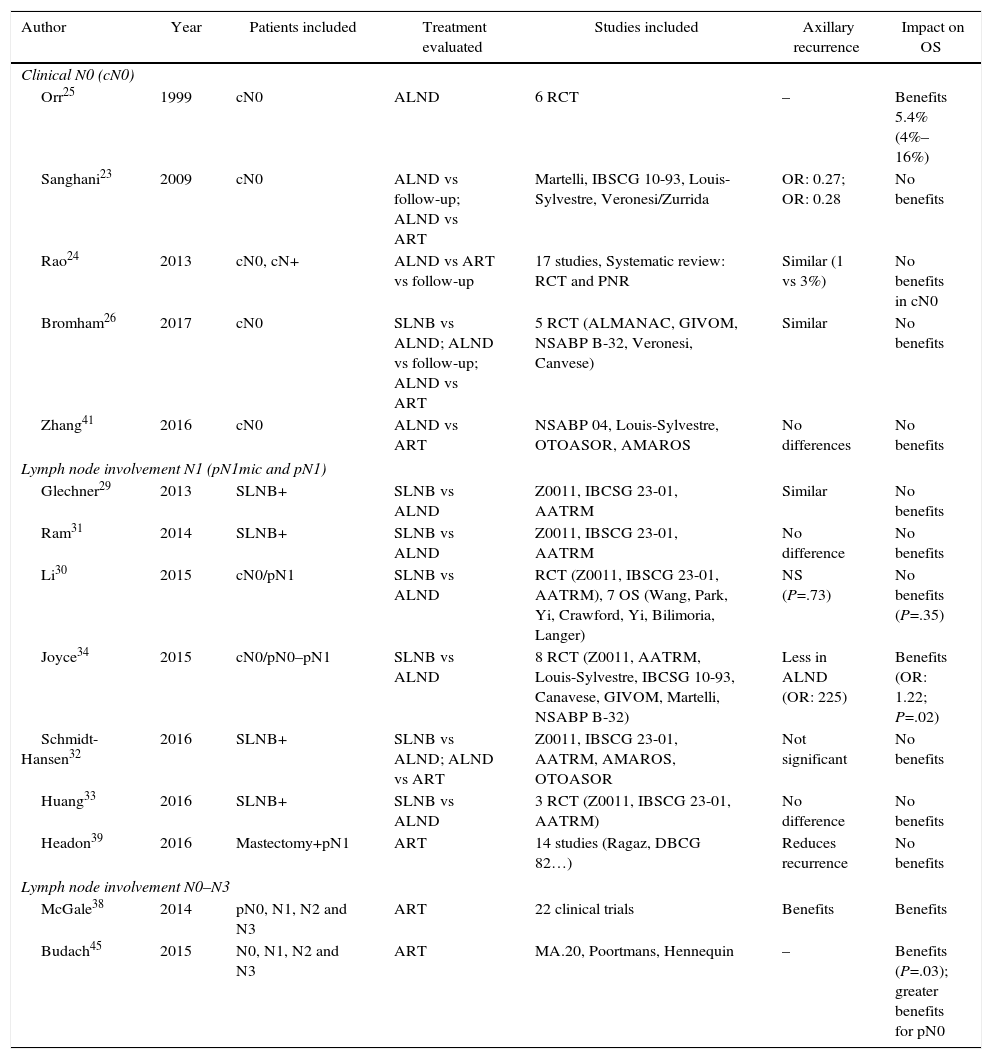
Three meta-analyses compiled the results of these studies.23–25 The meta-analysis by Sanghani et al.23 and Rao et al.24 demonstrated no benefits of ALND in the OS of patients without clinical involvement of the axilla (cN0). In contrast, the meta-analysis by Orr25 described a 5% benefit in the survival of women with ALND. However, this study presents 2 limitations: first, few patients with T1a tumors are included and, consequently, the extrapolation of these results may be inadequate, as a large number of patients had non-palpable tumors. Secondly, no patient received adjuvant chemotherapy, which could influence the reduction of risk evidenced in the meta-analysis (Table 2). Finally, the Cochrane review from 201726 showed similar OS in patients treated with SLNB and those with ALND.
Meta-Analyses Studying the Impact of Axillary Treatment (ART or ALND).
| Author | Year | Patients included | Treatment evaluated | Studies included | Axillary recurrence | Impact on OS |
|---|---|---|---|---|---|---|
| Clinical N0 (cN0) | ||||||
| Orr25 | 1999 | cN0 | ALND | 6 RCT | – | Benefits 5.4% (4%–16%) |
| Sanghani23 | 2009 | cN0 | ALND vs follow-up; ALND vs ART | Martelli, IBSCG 10-93, Louis-Sylvestre, Veronesi/Zurrida | OR: 0.27; OR: 0.28 | No benefits |
| Rao24 | 2013 | cN0, cN+ | ALND vs ART vs follow-up | 17 studies, Systematic review: RCT and PNR | Similar (1 vs 3%) | No benefits in cN0 |
| Bromham26 | 2017 | cN0 | SLNB vs ALND; ALND vs follow-up; ALND vs ART | 5 RCT (ALMANAC, GIVOM, NSABP B-32, Veronesi, Canvese) | Similar | No benefits |
| Zhang41 | 2016 | cN0 | ALND vs ART | NSABP 04, Louis-Sylvestre, OTOASOR, AMAROS | No differences | No benefits |
| Lymph node involvement N1 (pN1mic and pN1) | ||||||
| Glechner29 | 2013 | SLNB+ | SLNB vs ALND | Z0011, IBCSG 23-01, AATRM | Similar | No benefits |
| Ram31 | 2014 | SLNB+ | SLNB vs ALND | Z0011, IBSCG 23-01, AATRM | No difference | No benefits |
| Li30 | 2015 | cN0/pN1 | SLNB vs ALND | RCT (Z0011, IBSCG 23-01, AATRM), 7 OS (Wang, Park, Yi, Crawford, Yi, Bilimoria, Langer) | NS (P=.73) | No benefits (P=.35) |
| Joyce34 | 2015 | cN0/pN0–pN1 | SLNB vs ALND | 8 RCT (Z0011, AATRM, Louis-Sylvestre, IBCSG 10-93, Canavese, GIVOM, Martelli, NSABP B-32) | Less in ALND (OR: 225) | Benefits (OR: 1.22; P=.02) |
| Schmidt-Hansen32 | 2016 | SLNB+ | SLNB vs ALND; ALND vs ART | Z0011, IBSCG 23-01, AATRM, AMAROS, OTOASOR | Not significant | No benefits |
| Huang33 | 2016 | SLNB+ | SLNB vs ALND | 3 RCT (Z0011, IBSCG 23-01, AATRM) | No difference | No benefits |
| Headon39 | 2016 | Mastectomy+pN1 | ART | 14 studies (Ragaz, DBCG 82…) | Reduces recurrence | No benefits |
| Lymph node involvement N0–N3 | ||||||
| McGale38 | 2014 | pN0, N1, N2 and N3 | ART | 22 clinical trials | Benefits | Benefits |
| Budach45 | 2015 | N0, N1, N2 and N3 | ART | MA.20, Poortmans, Hennequin | – | Benefits (P=.03); greater benefits for pN0 |
SLNB: sentinel lymph node biopsy; RCT: randomized clinical trial; OS: observational studies; ALND: axillary lymph node dissection; NRP: non-randomized prospective study; ART: axillary radiotherapy; OS: overall survival.
Micrometastasis (pN1mic): Two CT11,12 compared ALND to observation in patients with micrometastatic involvement of the axilla in breast-conserving surgery and mastectomy. In both CT, the average 5-year follow-up did not show significant differences in DFS or OS between both groups, so the authors concluded that ALND can be omitted in women with micrometastatic involvement of the SLN (Table 1).
Macrometastasis (pN1): The ACOSOG-Z001113 trial is the only one that specifically analyzes the benefit of ALND in women with macrometastasis of the SLN. This study included women with up to 2 metastatic SLN (44.8% with micrometastasis) who underwent breast-conserving surgery and radiotherapy and were randomized to follow-up or ALND. With a mean follow-up of 9.25 years,27 the updated data of this CT show a similar incidence of axillary recurrence (SLNB: 1.5% and ALND: 0.5%) and OS in both groups, with no differences in regional recurrence between patients with radiotherapy of the 3 lymph node levels and those who only received tangential fields. The authors conclude that, in a group of women with early-stage breast cancer and SLN involvement, ALND can be omitted (Table 1). Some authors have indicated that this study presents certain limitations, among them the early finalization of the study (891 patients instead of the intended 1900), the majority of patients with luminal tumors (80%), no immunohistochemical study of the SLN and, in particular, the omission of the description of the lymph node radiotherapy fields. This last factor led the authors of the ACOSOG-Z001113 to review the planning of the radiotherapy fields used in the patients included in the study. In 2014, the authors published an article analyzing radiotherapy fields and found that 81.1% of patients only received breast radiation therapy and that there were no differences in the incidence of regional recurrence between patients with or without ART.28
Six meta-analyses29–34 have analyzed the impact of ALND in women with SLN metastasis (Table 2). Five of them29–33 determined that, in patients with clinically negative axillae and micrometastatic involvement of the SLN (pN1mic), suppression of ALND permits adequate local control without compromising OS. In contrast, the meta-analysis by Joyce et al.34 showed evidence of the benefit of ALND in terms of axillary recurrence and OS.
Axillary Radiotherapy in Patients With Lymph Node Involvement (N1–N3)Two CT, the DBCG 8235 and the Ragaz et al. trial,36,37 analyze the impact of ART in the survival of patients with breast cancer (Table 1). Both found a significant decrease in locoregional recurrences and distant metastases, which is seen in an increase in long-term OS in patients with metastasis in 4 or more lymph nodes and regional radiotherapy. The CT by Ragaz36,37 found the same benefit for patients with involvement of between 1 and 3 lymph nodes.
Two meta-analyses38,39 have analyzed the impact of lymph node radiotherapy in patients with axillary involvement. The study by McGale et al.,38 which includes 22 randomized CT, showed evident benefits in locoregional control and OS for 15 years in patients with axillary involvement (N1, N2, and N3) and associated radiotherapy after mastectomy. The meta-analysis by Headon et al.39 evaluated lymph node radiation therapy in patients with N1 involvement and mastectomy and showed decreased risk of locoregional recurrence, with a minimum impact on OS (Table 2).
Axillary Radiotherapy in Patients Without Clinical Lymph Node Involvement (cN0)Two studies5,40 analyze the effect of ART without clinical involvement of the axilla (cN0). The first of them, the NSABP B-04,5 did not show differences in the OS of women with or without axillary treatment. The study by Zurrida et al.40 included 435 women with T1 tumors and clinically negative axillae (cN0), no axillary surgery (no ALND or SLNB), randomized to follow-up or ART and only showed a slight increase in axillary recurrence in the group with no axillary treatment (1 vs 0.5%) and no impact on survival (Table 1). Likewise, the meta-analysis by McGale et al.38 did not find any benefits of axillary radiation therapy in N0 patients.
Axillary Radiation Therapy as an Alternative to Axillary Lymph Node Dissection in Patients With Metastatic Sentinel Lymph NodeTwo CT, the AMAROS14 and OTOASOR,15 have analyzed the impact of ART as an alternative to ALND in N1 patients (Table 1). Both CT studied the non-inferiority of lymph node radiation therapy versus ALND in patients with clinically negative axillae and metastatic involvement of the SLN. No significant differences were found in the axillary recurrences or in the 5-year OS between the two groups, with a lower rate of lymphedemas in women without ALND. The authors concluded that lymph node radiotherapy is a valid alternative to ALND in these patients.
The results of these 2 CT14,15 were included in the meta-analyses by Zhang et al.41 and Schmidt-Hansen et al.,32 showing similar DFS and OS in pN1 patients treated with ART and without ALND (Table 2).
Internal Mammary Chain RadiotherapyThree CT42–44 have evaluated the efficacy of internal mammary chain radiation (Table 1). The MA.2042 included patients with breast-conserving surgery, with N1 axillary involvement or no lymph node involvement and risk factors for local recurrence, who were randomized to lymph node radiotherapy (including the internal mammary chain) or follow-up. All patients with lymph node involvement underwent ALND. No significant differences were observed in OS after 10 years between the two groups, but there was a decrease in the rate of disease recurrence. The second of the CT, by Poortmans et al.,43 included more than 4000 patients with central or medial breast tumors in stages I, II or III, with ALND in cases of lymph node involvement, and analyzed the impact of radiotherapy on the internal mammary chain. The results of the study did not show differences in OS, although a decrease was observed in locoregional recurrences and distant metastasis in the irradiated group. Both studies concluded that the individualized selection of the therapeutic regimen is the key to improved survival. The multicenter CT by Hennequin et al.44 randomized patients with lymph node involvement (N1–N3) or medial tumors to radiation or no radiation of the internal mammary chain. After 10 years of follow-up, the authors found no benefit in the local control of the disease or OS.
The meta-analysis by Budach et al.45 (Table 2), which included these 3 CT, concluded that the irradiation of the internal mammary chain generates a certain benefit in OS, although after 10 years this benefit is minimal (1 vs 3.3%).
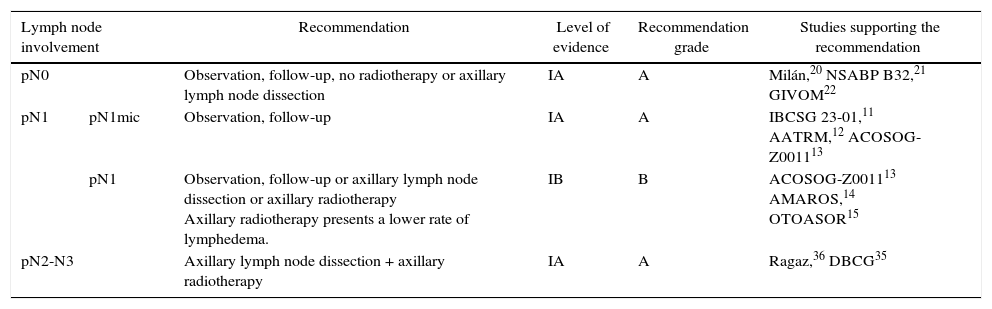
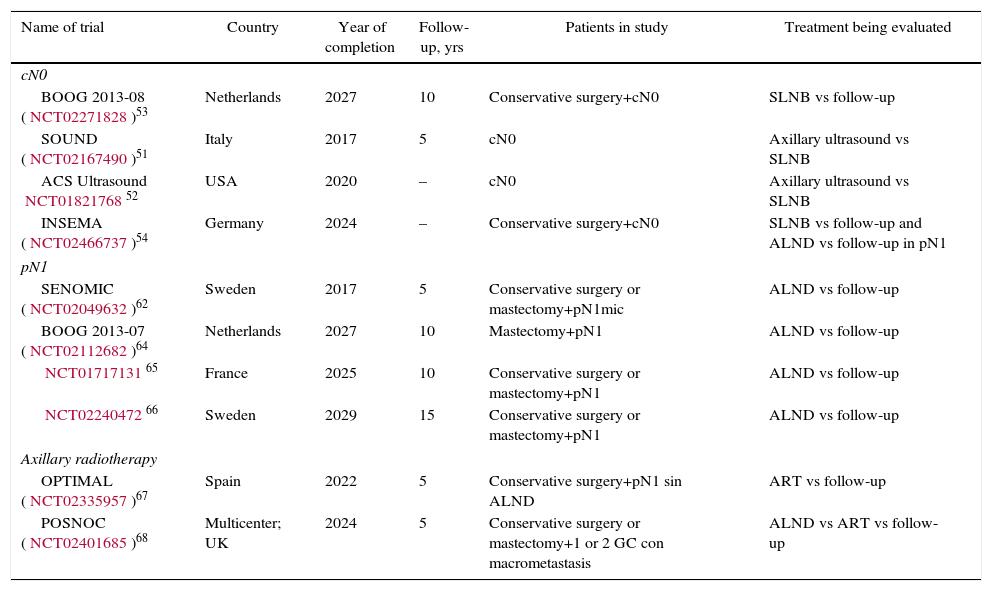
DiscussionSeveral CT have shown a risk of axillary recurrence between 19 and 37% in clinically negative axillae that do not receive treatment (no ALND or radiation therapy), which can be reduced to 0%–3.5% with either ALND or ART.46–49 These results show the importance of axillary evaluation, using either ultrasound or SLNB, to identify women with lymph node involvement but no clinical evidence who would benefit from axillary treatment. However, the trials completed prior to the introduction of SLNB5–10 showed no differences in the survival of patients with clinically negative axillae treated with ALND, ART or follow-up. The same is true for the meta-analyses23,24 that included these studies, in spite of residual axillary disease ranging from 21 to 40%. These CT5–10 are old, so their results are not presently applicable. Subsequently, the introduction of the SLNB technique has demonstrated the safety of suppressing ALND in patients with no metastatic involvement of the SLN. This staging method of stability does not compromise DFS or OS, in spite of 10% false negatives, and the morbidity rate is lower. These studies constitute the scientific basis for not treating the axilla (no ALND or ART) in women without metastatic involvement of the SLN (pN0) and this recommendation is collected in international clinical guidelines2,3,50 (Table 3). Currently, 4 CT in progress are evaluating the need for SLNB in patients with clinically negative axillae at diagnosis and will compare axillary staging by ultrasound versus SLNB.51–54 The results will be published between 2017 and 2027 (Table 4).
Axillary Treatment Recommendation According to Lymph Node Involvement.
| Lymph node involvement | Recommendation | Level of evidence | Recommendation grade | Studies supporting the recommendation | |
|---|---|---|---|---|---|
| pN0 | Observation, follow-up, no radiotherapy or axillary lymph node dissection | IA | A | Milán,20 NSABP B32,21 GIVOM22 | |
| pN1 | pN1mic | Observation, follow-up | IA | A | IBCSG 23-01,11 AATRM,12 ACOSOG-Z001113 |
| pN1 | Observation, follow-up or axillary lymph node dissection or axillary radiotherapy Axillary radiotherapy presents a lower rate of lymphedema. | IB | B | ACOSOG-Z001113 AMAROS,14 OTOASOR15 | |
| pN2-N3 | Axillary lymph node dissection + axillary radiotherapy | IA | A | Ragaz,36 DBCG35 | |
Clinical Trials Underway.
| Name of trial | Country | Year of completion | Follow-up, yrs | Patients in study | Treatment being evaluated |
|---|---|---|---|---|---|
| cN0 | |||||
| BOOG 2013-08 (NCT02271828)53 | Netherlands | 2027 | 10 | Conservative surgery+cN0 | SLNB vs follow-up |
| SOUND (NCT02167490)51 | Italy | 2017 | 5 | cN0 | Axillary ultrasound vs SLNB |
| ACS Ultrasound NCT0182176852 | USA | 2020 | – | cN0 | Axillary ultrasound vs SLNB |
| INSEMA (NCT02466737)54 | Germany | 2024 | – | Conservative surgery+cN0 | SLNB vs follow-up and ALND vs follow-up in pN1 |
| pN1 | |||||
| SENOMIC (NCT02049632)62 | Sweden | 2017 | 5 | Conservative surgery or mastectomy+pN1mic | ALND vs follow-up |
| BOOG 2013-07 (NCT02112682)64 | Netherlands | 2027 | 10 | Mastectomy+pN1 | ALND vs follow-up |
| NCT0171713165 | France | 2025 | 10 | Conservative surgery or mastectomy+pN1 | ALND vs follow-up |
| NCT0224047266 | Sweden | 2029 | 15 | Conservative surgery or mastectomy+pN1 | ALND vs follow-up |
| Axillary radiotherapy | |||||
| OPTIMAL (NCT02335957)67 | Spain | 2022 | 5 | Conservative surgery+pN1 sin ALND | ART vs follow-up |
| POSNOC (NCT02401685)68 | Multicenter; UK | 2024 | 5 | Conservative surgery or mastectomy+1 or 2 GC con macrometastasis | ALND vs ART vs follow-up |
Various studies55–60 have analyzed the clinical relevance of the micrometastatic involvement of the SLN and the need for ALND. Mittendorf et al.60 did not show differences in OS or DFS among patients with stage IA (pN0) and IB (pN1mi) breast cancer. Instead, the biological characteristics of the tumor, such as hormone receptors and tumor grade, were related to survival. In the same way, Giuliano et al.61 did not detect either a decrease in survival in those women with micrometastasis of the SLN detected by immunohistochemistry. The results of 3 CT (IBCSG 23-01,11 ATTRM12 and ACOSOG-Z001113), as well as various meta-analyses,29–33 recommend observation without ALND in patients with micrometastatic involvement of the SLN, either in conservative surgery or in mastectomy. Only one of the meta-analyses34 included in this review demonstrated ALND to be beneficial in this group of patients. However, this meta-analysis includes methodologically disparate CT comparing ALND with ART prior to the introduction of the SLNB and SLNB validation studies. Currently, American and European clinical guides1,50 recommend omitting axillary treatment (no ALND or ART) in patients with SLN micrometastasis (Table 3). The SENOMIC62 trial, whose results should be published this year, will show more evidence about the impact of suppressing ALND in patients with SLN micrometastasis and breast-conserving surgery or mastectomy (Table 4).
Patients with macrometastatic involvement of the axilla limited to 1–3 lymph nodes are currently the most controversial groups for axillary treatment. The main difficulty for a recommendation in these patients is their heterogeneity, since there are patients with 1–3 lymph nodes, with and without extracapsular involvement, tumors with adverse tumor biology and breast-conserving surgery or mastectomy. This variety of patients has not been adequately categorized in the CT and, for this reason, we lack a criterion that would allow us to identify which N1 patients are at high risk for axillary recurrence. The ACOSOG-Z001113 study included a sample of patients with predominance of luminal tumors, a large percentage of them only with micrometastasis, and it seems to indicate that in this group of patients the omission of ALND is safe in breast-conserving surgery. Other authors14,15 propose replacing ALND with ART in patients without clinical axillary involvement with SLN metastasis. However, both trials (AMAROS14 and OTOASOR15) did not include a control group without axillary treatment, which forces one to question the need for radiating the 3 axillary levels and supraclavicular area in all patients with metastatic SLN without ALND. An observational study of our center63 proposes treatment with ART in patients with macrometastatic SLN involvement without ALND who present other risk factors for regional recurrence (triple-negative tumors or HER2, lymphovascular invasion, high tumor grade, etc.). However, this study presents all the limitations of not being a randomized CT and its conclusions do not establish recommendations.
Therefore, there is presently not enough evidence to suppress the axillary treatment in these patients, but there also is no evidence to support the systematic indication of ALND or ART. The future of this discussion should be oriented toward the introduction of biological criteria in the decision-making process of axillary treatment, such as that done with gene platforms for the indication of systemic treatment. While awaiting this possibility, Huang et al.33 recommend including patient preference in the final decision. Currently, 3 CT64–66 are under way that will study the impact of ALND versus follow-up in patients with macrometastatic involvement of the SLN. Another 2 CT67,68 will analyze the impact of ART. The OPTIMAL67 trial includes patients with metastatic involvement of the SLN without ALND and randomizes them to ART or follow-up. The POSNOC68 assay includes patients with metastatic SLN involvement and randomizes them for observation, ALND or ART. The results of these trials will be available between 2022 and 2027 (Table 4). Until these studies are published, and based on the previously raised premises, it seems necessary for each case to be individualized by a multidisciplinary committee, in which the patient's opinion could be incorporated. We propose suppressing axillary treatment (no ALND or ART) in women with low risk for locoregional recurrence and recommend ART in patients with risk factors for locoregional recurrence.
Finally, 2 CT35–37 justify the association of ALND and ART in patients with metastasis in 4 or more axillary lymph nodes. Although these trials are old and patients did not receive specific systemic treatments (antibodies), clinical guidelines based on these studies recommend ALND and lymph node radiotherapy (axillary and supraclavicular) in patients with N2–N3 lymph node involvement (Table 3). On the contrary, although the irradiation of the internal mammary chain decreases the risk of local relapse, it has not shown a benefit in OS.42–44 Therefore, the internal mammary chain should not be included in the radiotherapy fields (Table 3).
This review presents several limitations. First, the oldest studies included patients with less effective adjuvant treatment compared to the most recent trials. This is especially important in the risk for locoregional recurrence of HER2 tumors without biological therapy. Second, many studies do not contemplate the categorization of risk factors for recurrence and the biological characteristics of the disease, which prevents estimating the effect of the treatments. Finally, the lack of statistical analysis of our review does not allow the impact of the proposed recommendations to be established.
In conclusion, this systematic review establishes the suppression of axillary treatment in women with breast cancer without pathological lymph node involvement (pN0) or with micrometastatic involvement of the SLN, since they do not benefit from axillary treatment (no ART nor ALND). In contrast, patients with extensive axillary disease (N2 and N3) benefit from ALND and ART to improve their OS and DFS. Patients with macrometastatic axillary disease (pN1) constitute a heterogeneous group that requires individualized analysis of risk factors to determine optimal axillary treatment. The recommendations in this group of patients will be defined by clinical trials that are currently underway, whose results will become available in the next decade.
Conflict of InterestsThere are no conflicts of interests.
Please cite this article as: García Novoa A, Acea Nebril B. Estado actual del tratamiento de la axila en la cirugía primaria del cáncer de mama: revisión sistemática de su impacto en la supervivencia. Cir Esp. 2017;95:503–512.