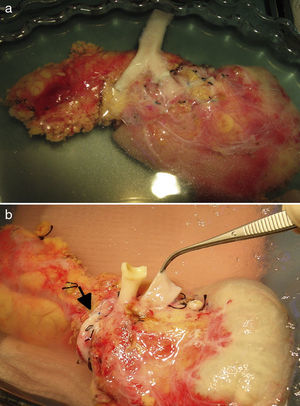
In the 50 years since the first pancreas transplant performed at the University of Minnesota, the surgical techniques employed have undergone many modifications. Techniques such as retroperitoneal graft placement have further improved the ability to reproduce the physiology of the “native” pancreas. We herein present our experience of a modified technique for pancreatic transplant, with the organ placed into a fully retroperitoneal position with systemic venous and enteric drainage of the graft by duodeno-duodenostomy.
MethodsAll pancreas transplantations performed between May 2016 and January 2017 were prospectively entered into our transplant database and retrospectively analyzed.
ResultsA total of 10 transplants were performed using the retroperitoneal technique (6 men: median age of 41 years [IQR 36–54]). Median cold ischemia time was 10.30h [IQR 5.30–12.10]. The preservation solution used was Celsior (n=7), IGL-1 (n=2), and UW (n=1).
No complications related to the new surgical technique were identified. In one patient, transplantectomy at 12h was performed due to graft thrombosis, probably related to ischemic conditions from a donor with prolonged cardio-respiratory arrest. Another procedure was aborted without completing the graft implant due to an intraoperative immediate arterial thrombosis in a patient with severe iliac atheromatosis. No primary pancreas non-function occurred in the remaining 8 patients. The median hospital stay was 13.50 days [IQR 10–27].
ConclusionsRetroperitoneal graft placement appears feasible with easy access for dissection the vascular site; comfortable technical vascular reconstruction; and a decreased risk of intestinal obstruction by separation of the small bowel from the pancreas graft.
Después de transcurridos más de 50 años desde el primer trasplante de páncreas realizado en la Universidad de Minnesota, las técnicas quirúrgicas empleadas han experimentado muchas modificaciones. La colocación del injerto en posición retroperitoneal reproduce la fisiología del páncreas «nativo». El objetivo del estudio es presentar la experiencia de la aplicación de una técnica modificada, con el injerto pancreático en posición retroperitoneal, con drenaje venoso sistémico y duodenoduodenostomía para el drenaje entérico.
MétodosAnálisis retrospectivo de los trasplantes de páncreas realizados entre mayo de 2016 y enero de 2017 en una sola institución.
ResultadosSe incluyen un total de 10 trasplantes (6 hombres: mediana de edad de 41 años [IQR 36-54]). El tiempo de isquemia fría fue de 10,30 h [IQR 5,30-12,10]. La solución de preservación utilizada fue Celsior (n=7), IGL-1 (n=2) y UW (n=1).
No se han identificado complicaciones relacionadas directamente con la posición retroperitoneal y la derivación duodenoduodenal. Un paciente requirió trasplantectomía a las 12 h por trombosis del injerto proveniente de un donante con paro cardiorrespiratorio prolongado. Otro procedimiento fue abortado debido a una trombosis arterial intraoperatoria en un paciente con ateromatosis ilíaca grave. Los restantes pacientes presentaron una correcta función del injerto, sin requerimientos de insulina. La estancia hospitalaria fue de 13,50 días (IQR 10-27).
ConclusionesLa colocación del injerto retroperitoneal es una técnica factible, que permite un fácil acceso para la disección de los vasos y posterior reconstrucción vascular y que minimiza, a su vez, el riesgo de oclusión intestinal.
Currently, pancreas transplantation is the only therapy that has demonstrated effectiveness for correcting metabolic control. It is considered the gold-standard treatment for type 1 diabetes mellitus in selected cases.
In 1966, William Kelly and Richard Lillehei performed the first pancreas transplantation in the world at the University of Minnesota.1–3 This initial experience anticipated the future destiny of pancreatic transplantation: functioning grafts with diabetes correction and no need for insulin in the long term,4,5 but risks included surgical6–13 and immunological14 complications. In this context, refinements and advances in the surgical technique have often been preceded by improvements in graft preservation,15–18 immunosuppression,4,5 and antimicrobial prophylaxis.4 However, after more than 50 years of history, no surgical technique has become standardized among the different transplant groups.
Most likely, the position of the pancreatic graft is what determines the type of vascular anastomoses and exocrine drainage. Traditionally, the intraperitoneal position has been preferred by most medical centers. In the last decade, however, various authors have advocated the placement of the graft in the retroperitoneal area, which is a more physiological position.
If we focus on the drainage method of exocrine secretions, the urinary diversion was most frequently used until the mid-1990s, when it was replaced by enteric drainage,19 which is considered the current standard. In the most common method, the anastomosis is performed between the graft duodenum and the jejunum of the recipient, with the graft in intraperitoneal position. The enteric anastomosis can be performed to the proximal jejunum20–22 or to the distal ileum,23 and can be either end-to-end,24,25 end-to-side26,27 or side-to-side.28–32 The use of direct anastomosis is currently more frequent21,33–36 than Roux-en-Y anastomosis.28,29,31,32,36 However, Boggi et al.28,29 have shown excellent results with side-to-side Roux-en-Y duodenojejunostomy, with retroperitoneal graft placement and portal venous drainage. Techniques using exocrine drainage to the stomach have also been described.37,38
A few surgical innovations have been recently described related with graft placement, type of intestinal anastomosis, and their contribution to improved graft function. Duodenoduodenostomy (DD) is an interesting option for the drainage of gastrointestinal secretions, when the pancreas is placed behind the right colon and is oriented cranially.28,29,39–46
Indeed, there is a lack of consensus between experts in terms of the “ideal” surgical technique. Correct long-term graft function is only possible if the transplantation is technically successful. There is a need for a more objective evaluation and standardized surgical techniques for pancreatic transplantation.
For this reason, and after more than 30 years of experience in intraperitoneal pancreas transplantation at our hospital, the objective of this article is to present a modification of the technique with the placement of the pancreatic graft in a retroperitoneal position, with systemic venous drainage and DD for enteric drainage. We will analyze the potential advantages, while focusing on postoperative complications.
MethodsWe have retrospectively analyzed pancreas transplantations performed at a single hospital between May 2016 and January 2017, collected prospectively in a database. The indications for the pancreas transplantation were patients affected by diabetes mellitus who met the inclusion criteria according to the protocol established at our institution.47
Induction therapy was carried out with rabbit antithymocyte globulin (Timoglobulina®; total dose 6mg/kg) and maintenance with a calcineurin inhibitor (tacrolimus), an antimetabolite (mycophenolate-mofetil or mycophenolate sodium) and prednisone.
Antithrombotic prophylaxis was based on intravenous sodium heparin before graft reperfusion, followed by low molecular weight heparin and aspirin in the immediate postoperative period. Vancomycin and ertapenem were used as antibiotic prophylaxis in the perioperative period, and valganciclovir and co-trimoxazole during the first months post-transplantation.
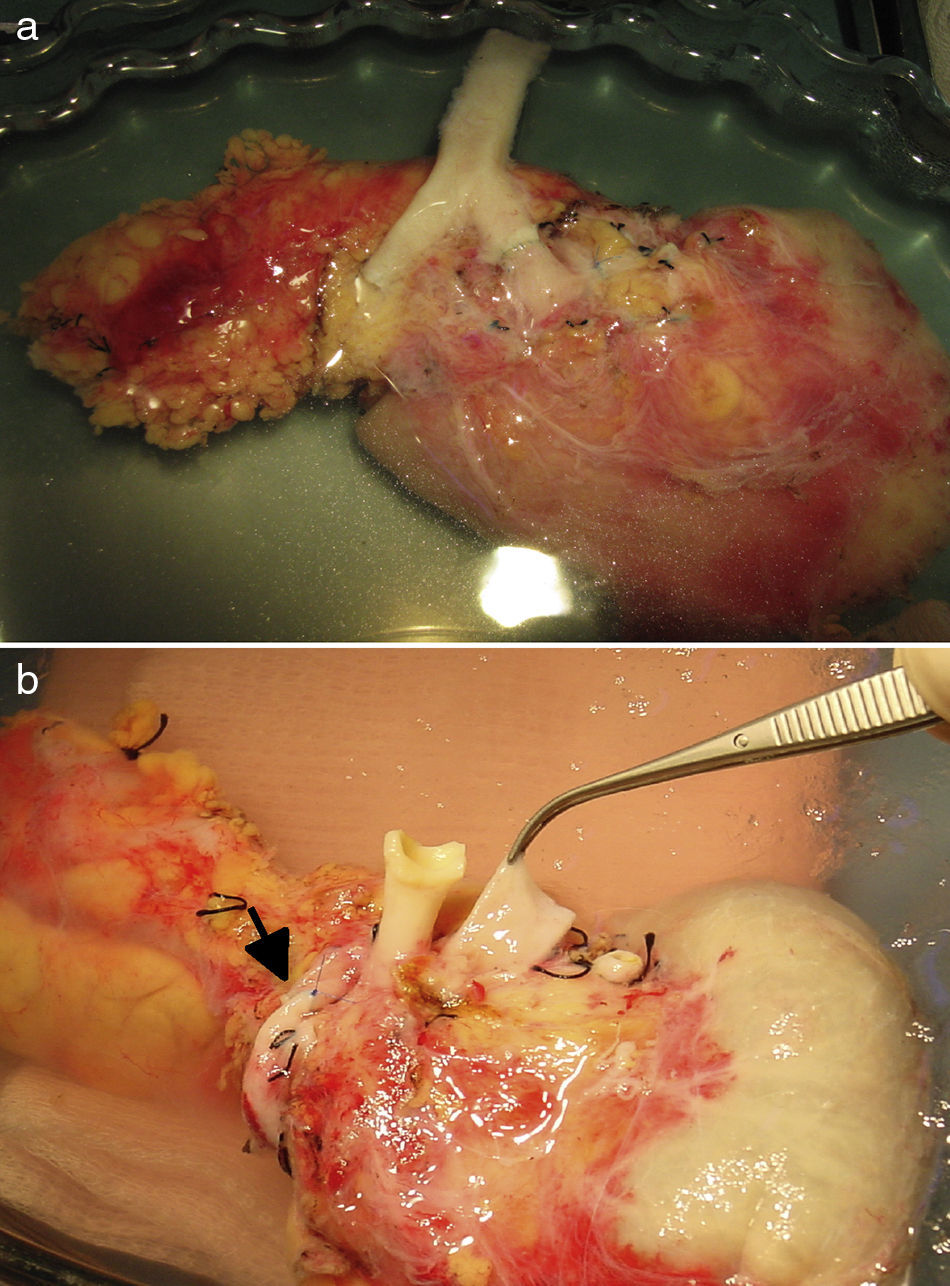
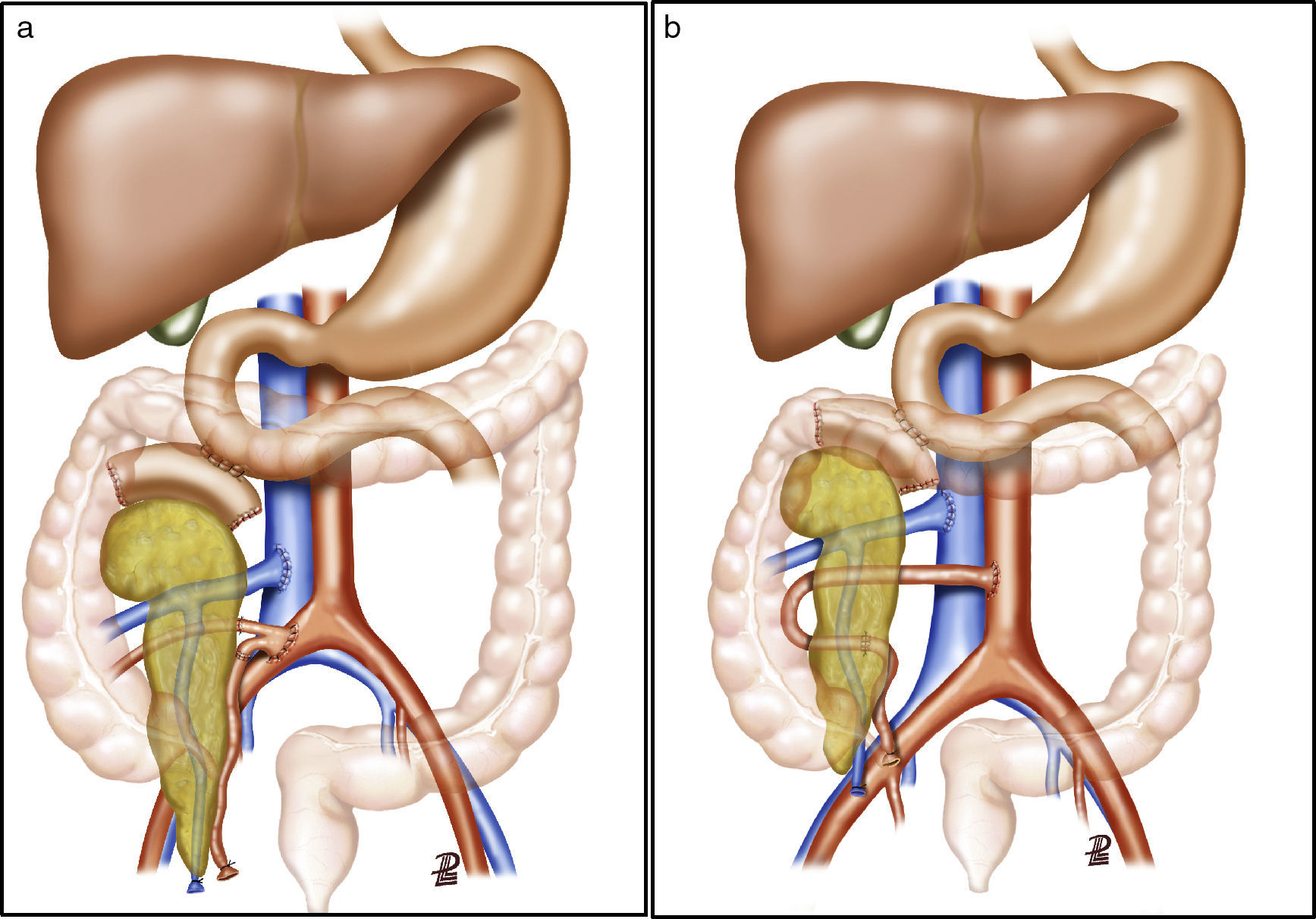
Surgical TechniqueBench SurgeryFor the vascular reconstruction of the pancreatic graft, 2 different techniques were used:
Surgical Technique in the RecipientIn the case of simultaneous pancreas and kidney transplantation, the pancreas graft is implanted before the kidney. The surgical technique utilized for the implantation of the renal graft does not differ from that used in isolated kidney transplantation, placed extraperitoneal in the left iliac fossa.
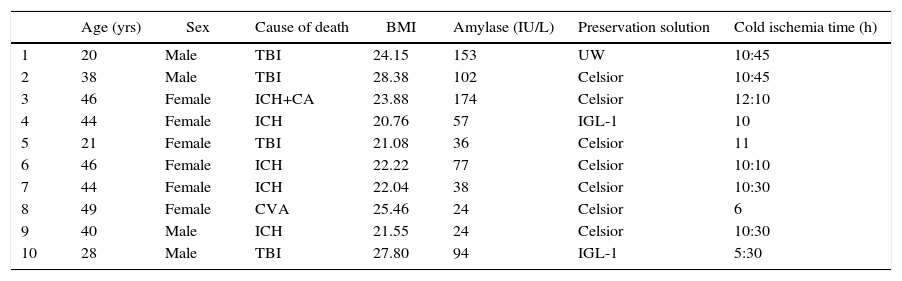
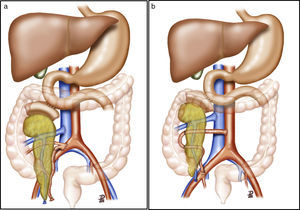
After making a midline laparotomy incision, the right colon is mobilized and the Kocher maneuver is completed. The vena cava is dissected along its length toward the right iliac vein. The primitive iliac artery together with the external iliac artery and the hypogastric artery are identified with vessel loops. The pancreas is placed with the head of the pancreas and the duodenum in cranial position, and the tail in caudal position. First, end-to-lateral venous anastomosis is created between the portal vein of the graft and the vena cava, with 2 continuous Prolene® 5/0 sutures. Afterwards, end-to-side arterial anastomosis is done with continuous Prolene® 5/0 sutures, between the segment of the primitive iliac artery of the graft (Fig. 2a) or the superior mesenteric artery (Fig. 2b), depending on the bench surgery performed, with the right primitive iliac artery of the recipient. Afterwards, the clamps are released sequentially from the vascular anastomoses, first the vein and then the artery, to reperfuse the graft.
(a) Pancreas transplantation with enteric drainage of the exocrine secretions to the native duodenum, systemic venous drainage and arterial reconstruction of the pancreas using a Y arterial graft; (b) pancreas transplantation with enteric drainage of the exocrine secretions to the native duodenum, systemic venous drainage and arterial reconstruction of the pancreas using end-to-end splenomesenteric anastomosis. The diagram shows the arterial anastomosis of the graft to the aorta. In our series, the anastomosis was done to the right primitive iliac artery.
The pancreatic exocrine secretions are drained to the native duodenum. After correct mobilization of the recipient duodenum, the side-to-side anastomosis is created (2.5–3cm) between the duodenum of the graft and the 2nd–3rd portion of the duodenum of the recipient in 2 planes, one internal for the mucosa with absorbable material (vicryl 3/0) and another external seromuscular, with non-absorbable suture (silk 3/0). Afterwards, the right colon is replaced in its usual position, and the pancreas is rendered unmovable.
Immediate Postoperative ControlDoppler ultrasound was systematically performed 24h post-transplantation and before hospital discharge. In cases of fever, abdominal pain or pancreatic graft dysfunction, abdominal CT scans were ordered. Likewise, between the 3rd and 5th days post-op, bowel transit tests were done to check the DD for leaks before beginning oral intake.
Statistical AnalysisA descriptive analysis of patient characteristics was performed. The quantitative variables have been expressed with median and range. The analysis was calculated with SPSS statistical software (SPSS 20.0, 1989–1995; Chicago, IL, USA).
The study was approved by the Ethics Committee at our institution.
ResultsDuring the period described, a total of 10 pancreas transplantations were performed by placing the graft in the retroperitoneal position. In all cases, the indication was type 1 diabetes mellitus.
In most patients, the patient was simultaneously transplanted with the kidney (n=9), and both grafts came from the same cadaveric donor. In one case, the pancreas transplantation was done in a patient who, 20 months earlier, had received a kidney from a living ABO-incompatible donor.
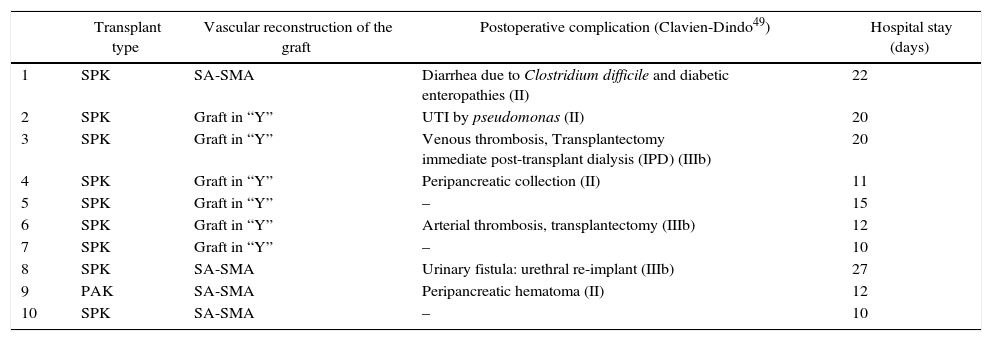
The variables related with the donor corresponding to each study case are described in Table 1. The median age was 42 years (20–49), with a BMI of 23.05 (20.76–28.38). Cold ischemia time was 10.30h (5.30–12.10), and the preservation solutions used for graft perfusion were Celsior (n=7), IGL-1 (n=2) and UW (n=1).
Donor Characteristics.
| Age (yrs) | Sex | Cause of death | BMI | Amylase (IU/L) | Preservation solution | Cold ischemia time (h) | |
|---|---|---|---|---|---|---|---|
| 1 | 20 | Male | TBI | 24.15 | 153 | UW | 10:45 |
| 2 | 38 | Male | TBI | 28.38 | 102 | Celsior | 10:45 |
| 3 | 46 | Female | ICH+CA | 23.88 | 174 | Celsior | 12:10 |
| 4 | 44 | Female | ICH | 20.76 | 57 | IGL-1 | 10 |
| 5 | 21 | Female | TBI | 21.08 | 36 | Celsior | 11 |
| 6 | 46 | Female | ICH | 22.22 | 77 | Celsior | 10:10 |
| 7 | 44 | Female | ICH | 22.04 | 38 | Celsior | 10:30 |
| 8 | 49 | Female | CVA | 25.46 | 24 | Celsior | 6 |
| 9 | 40 | Male | ICH | 21.55 | 24 | Celsior | 10:30 |
| 10 | 28 | Male | TBI | 27.80 | 94 | IGL-1 | 5:30 |
CVA: ischemic cerebrovascular accident; ICH: intracranial hemorrhage; IGL-1: Institut Georges Lopez 1; BMI: body mass index; CA: cardiac arrest; TBI: traumatic brain injury; UW: University of Wisconsin.
As for the recipients, 6 men and 4 women with a median age of 41 years (36–54) and a BMI of 22 (17.30–25.28) were included. The duration of the pre-transplantation diabetes was 29.50 years (19–44). The duration of dialysis was 18.50 months (3–62); hemodialysis (n=6), peritoneal dialysis (n=2) and pre-dialysis (n=1).
Table 2 demonstrates the variables related with the surgical intervention, such as the type of vascular reconstruction of the graft, as well as postoperative complications.
Recipient Characteristics.
| Transplant type | Vascular reconstruction of the graft | Postoperative complication (Clavien-Dindo49) | Hospital stay (days) | |
|---|---|---|---|---|
| 1 | SPK | SA-SMA | Diarrhea due to Clostridium difficile and diabetic enteropathies (II) | 22 |
| 2 | SPK | Graft in “Y” | UTI by pseudomonas (II) | 20 |
| 3 | SPK | Graft in “Y” | Venous thrombosis, Transplantectomy immediate post-transplant dialysis (IPD) (IIIb) | 20 |
| 4 | SPK | Graft in “Y” | Peripancreatic collection (II) | 11 |
| 5 | SPK | Graft in “Y” | – | 15 |
| 6 | SPK | Graft in “Y” | Arterial thrombosis, transplantectomy (IIIb) | 12 |
| 7 | SPK | Graft in “Y” | – | 10 |
| 8 | SPK | SA-SMA | Urinary fistula: urethral re-implant (IIIb) | 27 |
| 9 | PAK | SA-SMA | Peripancreatic hematoma (II) | 12 |
| 10 | SPK | SA-SMA | – | 10 |
AE: splenic artery; SMA: superior mesenteric artery; EGD: early graft dysfunction of renal transplant; UTI: urinary tract infection; PAK: pancreas after kidney transplantations; SPK: simultaneous pancreas/kidney transplantation.
No complications related to the retroperitoneal positioning of the graft have been identified. One patient required transplantectomy 12h after surgery for venous thrombosis of the graft, which had come from a donor with prolonged cardiorespiratory arrest (40min) and an excessively long ischemia period. In this case, the duodenotomy was repaired in 2 layers, with no later complications. Another procedure was aborted (without completing the intestinal anastomosis) due to immediate intraoperative arterial thrombosis at the time of reperfusion in a patient with severe iliac atheromatosis. The remaining 8 patients presented correct function of the pancreatic graft without the need for insulin. The hospital stay was 13.50 days (10–27).
The retroperitoneal location did not impede taking ultrasound-guided percutaneous graft biopsies. During the study period, a total of 6 biopsies were carried out in 4 patients transplanted with the new technique (protocol n=4; indication n=1; post-rejection follow-up n=1). A sample was obtained for diagnosis in all procedures, without any complications.
After a median follow-up of 5.83 months (1.67–9.73), patient survival was 100% and graft survival 80%.
DiscussionAlthough pancreas transplantation is characterized by involving one of the solid organs with the greatest incidence of surgical complications, it is currently the best method for restoring euglycemia in patients with diabetes mellitus type 1 and chronic kidney disease, and clearly offers improved quality of life.
The experience of our hospital in pancreatic transplantation is extensive, with more than 30 years of history and more than 500 transplantations to date. In an attempt to improve the results related to postoperative morbidity, the positioning of the pancreatic graft at the retroperitoneal level has been raised. This is added to the decision to derive exocrine secretions to the native duodenum of the recipient. In this article, we present the results of the first 10 cases performed with said technique.
In an attempt to improve the results related with postoperative morbidity, the retroperitoneal placement of the pancreatic graft has been proposed. Likewise, it has been decided to divert exocrine secretions to the native duodenum of the recipient. In this article, we present the results of the first 10 cases carried out with this technique.
The retroperitoneal position of the graft imitates the “physiological” position of this organ. Once the right colon is mobilized, the vascular structures are completely exposed, which technically simplifies completion of the anastomoses. In addition, another objective is to avoid torsion of the graft vessels. The creation of venous anastomoses to the vena cava of the recipient allows the duodenum of the donor and recipient to be in close proximity: a priori, this minimizes the percentage of graft thrombosis. In the present series, complete thrombosis of the graft requiring transplantectomy was attributed to ischemia-reperfusion phenomena of a suboptimal graft. The second case of graft loss was a consequence of a technical problem during arterial anastomosis in the context of important arterial atheromatosis. Walter et al.6 compared a series of 125 cases in which they performed retroperitoneal DD, in 116 of which intraperitoneal duodenojejunostomy (DJ) was performed. In this series, the main cause of graft loss was thrombosis (9.5%) with 5 cases (4%) in the DD group and 18 cases (15.5%) in the DJ group. On the other hand, in the series published by Gunasekaran et al.,44 more thromboses were observed in the DD group (9.5%) than in the DJ group (0%).
Another of the advantages lies in anatomical exposure, which differs from intraperitoneal placement, where the potential space between vascular and enteric anastomosis can serve as a defect through which the native intestine can herniate and lead to intestinal occlusion. In addition, chemical pancreatitis, due to the inevitable ischemia-reperfusion phenomena, contributes to paresis, which in turn causes postoperative paralytic ileus. In the present series, all patients presented rapid postoperative recovery, with no oral tolerance problems. Boggi et al.,48 in a series of 177 transplants, published 0.6% rate of intestinal obstruction as a cause of re-laparotomy. Walter et al.46 presented a total of 1.7% in DJ versus 0% in DD.
In the case of patients with indication for pancreas re-transplantation or patients who have had previous abdominal surgery, the retroperitoneal approach combined with DD also represents a valid alternative to take into account.
One of the controversial points related with DD focuses on cases of dehiscence of the intestinal anastomosis or in the cases of transplantectomy, due to the difficult duodenal repair in both scenarios.39–41,44,45 The patient of our series that required transplantectomy did not present any type of adverse event after repairing the duodenotomy. The German group with most experience in this type of anastomosis,46 along with other authors,44 consider that DD is a feasible and safe technique, with no increased intestinal complications. In addition, retroperitoneal placement can facilitate conservative treatment of enteric leaks in selected cases, due to the lower probability of intraperitoneal contamination.
Another concern that happens in this context is how to diagnose graft rejection. Doppler ultrasound with biopsy is the method of choice at our medical center. In the case of intraperitoneal placement of the graft, it is sometimes not possible to access the pancreas due to technical difficulties secondary to the presence of intestinal loops in the puncture site. The DD can provide an additional route, through the endoscopic approach, to view the mucosa of the donor's duodenum, as well as to take biopsies. Recently, Horneland et al.43 have published the first series of patients who underwent pancreatic graft biopsy performed by endoscopy through the DD. In our series of 10 patients, until now biopsies have been taken with no problems using ultrasound in the cases indicated. The DD also allows us to expand the range of therapeutic options in the event of exocrine leakage by placing stents in the pancreatic duct. Furthermore, it provides the capability to visualize the graft through simple gastroscopy in the case of intestinal hemorrhage in order to achieve hemostasis.
In conclusion, the retroperitoneal placement of the graft with diversion of the exocrine secretions from the graft to the native duodenum of the recipient is a feasible and safe technique, with potential advantages over the intraperitoneal position in terms of easy access for the dissection of the vessels and subsequent vascular reconstruction, lower risk of bowel obstruction, easy endoscopic access for taking of graft biopsies and for therapeutic purposes, if required.
It is necessary to increase the number of cases in our series to be able to obtain long-term patient and graft survival results, as it is currently considered by our group to be the surgical technique of choice in pancreas transplantation.
Authorship/CollaborationsJoana Ferrer: Study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Víctor Molina: Data collection, critical review and approval of the final version.
Ramón Rull: Data collection, critical review and approval of the final version.
Miguel Ángel López-Boado: Data collection, critical review and approval of the final version.
Santiago Sánchez: Data collection, critical review and approval of the final version.
Rocío García: Data collection, critical review and approval of the final version.
M. José Ricart: Analysis and interpretation of the results, critical review and approval of the final version.
Pedro Ventura-Aguiar: Analysis and interpretation of the results, critical review and approval of the final version.
Ángeles García-Criado: Data collection, critical review and approval of the final version.
Enric Esmatjes: Data collection, critical review and approval of the final version.
Josep Fuster: Analysis and interpretation of the results, article composition, critical review and approval of the final version.
Juan Carlos Garcia-Valdecasas: Study design, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Conflict of InterestThe authors have no conflict of interests to declare.
The authors would like to thank the Radiology Department at the Hospital Clínic for their constant contribution to the pre-transplantation study and postoperative follow-up, as well as their incalculable help to make this article possible.
Please cite this article as: Ferrer J, Molina V, Rull R, López-Boado MÁ, Sánchez S, García R, et al. Trasplante de páncreas: ventajas de la posición retroperitoneal del injerto. Cir Esp. 2017;95:513–520.
This study was presented in video format at the 12th Biennial E-AHPBA Congress 2017 of the European-African Hepato-Pancreato-Biliary Association, held May 23–26, 2017 in Mainz (Germany) with the title Pancreas transplantation: A retroperitoneal graft placement.
This study has also been accepted as an oral communication at the 16th International Conference of the International Pancreas and Islet Transplant Association (IPITA), held June 20–23, 2017 in Oxford (United Kingdom), under the title Pancreas transplantation: Advantages of a retroperitoneal graft position.