The role that self-expanding stents play in the treatment of dehiscence after transthoracic esophagectomy is not well defined and controversial. Our aim is to describe the experience in a tertiary care hospital using these devices for treating dehiscence after Ivor Lewis esophagectomy.
MethodsDescriptive observational study of patients who suffered anastomotic dehiscence after a transthoracic esophagectomy, and especially those treated with stents, in the period between 2011 and 2016 at our hospital.
ResultsTen patients (11.8%) presented anastomotic dehiscence. Eight patients received stents, one of them died due to causes unrelated to the device. Stent migration was observed in one case, and the devices were maintained an average of 47.3 days. The stent was not effective only in one patient who suffered early dehiscence due to acute ischemia of the stomach. The two patients who did not receive stents died after reoperation.
ConclusionsStents are safe and effective devices that did not associate mortality in our series. They are especially indicated in intermediate or late-onset dehiscence and in fragile patients. The use of stents, together with mediastinal and pleural drainage, avoid reoperations with morbidity and mortality. Therefore, stents should be part of the usual therapeutic arsenal for the resolution of most suture dehiscences after Ivor Lewis esophagectomy. Randomized prospective studies would help to more precisely determine the role played by these devices in the treatment of dehiscence after transthoracic esophagectomy.
El papel que desempeñan las endoprótesis autoexpandibles en el tratamiento de las dehiscencias tras la esofagectomía transtorácica no está bien definido y resulta controvertido. Nuestro objetivo es mostrar la experiencia en un hospital de tercer nivel con el empleo de estos dispositivos en las dehiscencias tras la esofagectomía de Ivor Lewis.
MétodosEstudio observacional descriptivo de los pacientes que han presentado una dehiscencia de anastomosis tras una esofagectomía transtorácica y, en especial, de aquellos tratados mediante endoprótesis, en el periodo comprendido entre 2011 y 2016 en nuestro centro hospitalario.
ResultadosDiez pacientes (11,8%) presentaron una dehiscencia anastomótica, 8 de los cuales recibieron endoprótesis. Un paciente portador de endoprótesis falleció por causas ajenas a la misma. En un paciente se objetivó migración del dispositivo, manteniéndose una media de permanencia de 47,3 días. La prótesis no fue efectiva en un paciente que tuvo una dehiscencia precoz por isquemia aguda gástrica. Fallecieron los 2 pacientes que no recibieron endoprótesis después de la reintervención.
ConclusionesLas endoprótesis son dispositivos seguros y efectivos que no asocian mortalidad en nuestra serie. Están especialmente indicadas en dehiscencias intermedias o tardías y en pacientes frágiles, pues, junto con el drenaje mediastínico y pleural, evitan reintervenciones gravadas con morbimortalidad. Por tanto, las endoprótesis deben formar parte del arsenal terapéutico habitual para la resolución de la mayoría de las dehiscencias de sutura tras la esofagectomía de Ivor Lewis. La puesta en marcha de estudios prospectivos aleatorizados ayudaría a determinar con mayor precisión el papel que desempeñan estos dispositivos en el tratamiento de las dehiscencias tras una esofagectomía transtorácica.
Dehiscence of the esophagogastric anastomosis is the most feared surgical complication after Ivor Lewis esophagectomy. Leakage of saliva, acid and bile secretions or food into the mediastinum causes serious infection and triggers a systemic inflammatory reaction with high associated mortality rates.1 Surgical reoperation to drain the contaminated pleural cavity and repair the anastomotic defect has been standard treatment for decades, but the results have been disparate. More recently, the management of these patients has been modified with the use of diagnostic endoscopy to determine the presence of ischemia in the gastroplasty as well as the dimensions and location of the defect, therapeutic endoscopy for the placement of self-expanding stents, and imaging-guided drainage of mediastinal and/or pleural collections. However, the use of stents in this clinical context has not been generalized, as could be expected, and this has become a subject of debate and discussion among esophagogastric surgeons.2,3 Our objective is to describe the results obtained in our hospital through the use of fully coated self-expanding metal stents and image-guided drainage in the treatment of esophagogastric dehiscence after the Ivor Lewis procedure.
MethodsFrom January 2011 to December 2016, we performed 85 Ivor Lewis procedures for esophageal cancer and cancer of the esophagogastric union at the Hospital Clínico Universitario Virgen de la Arrixaca (Murcia, Spain). Details from the patient file as well as the complementary explorations of each patient were discussed in a multidisciplinary committee including medical and radiation oncologists, pathologist, endoscopist, nutritionist, nuclear medicine specialist and surgeons. Out of the 85 patients operated on, 11 were women and 74 men, with an average age of 55.7 years (33–86). All patients except 9 received neoadjuvant treatment with chemotherapy and radiotherapy (CROSS study4) or perioperative chemotherapy (MAGIC study5). The details regarding the surgical technique as well as the perioperative management have been detailed in previous publications.6 According to the time of presentation, the dehiscences were classified as early (1st to 3rd postoperative day), intermediate (4th to 7th postoperative day) or late (after the 8th day).7 The stents were inserted under sedation by expert endoscopists, with the presence of an anesthesiologist in the operating room or in a specialized room of the Endoscopy Unit. The lengths of the devices varied between 18 and 24cm and all were fully coated metal prostheses (Hanarostent®-M.I. Tech., Seoul, Republic of Korea). In 2 cases, we affixed the prostheses with metal clips. We collected data for morbidity, in-hospital and 90-day mortality, hospital stay, the number of prostheses applied and radiological drains used, as well as the total number of endoscopies performed and the reason for performing them.
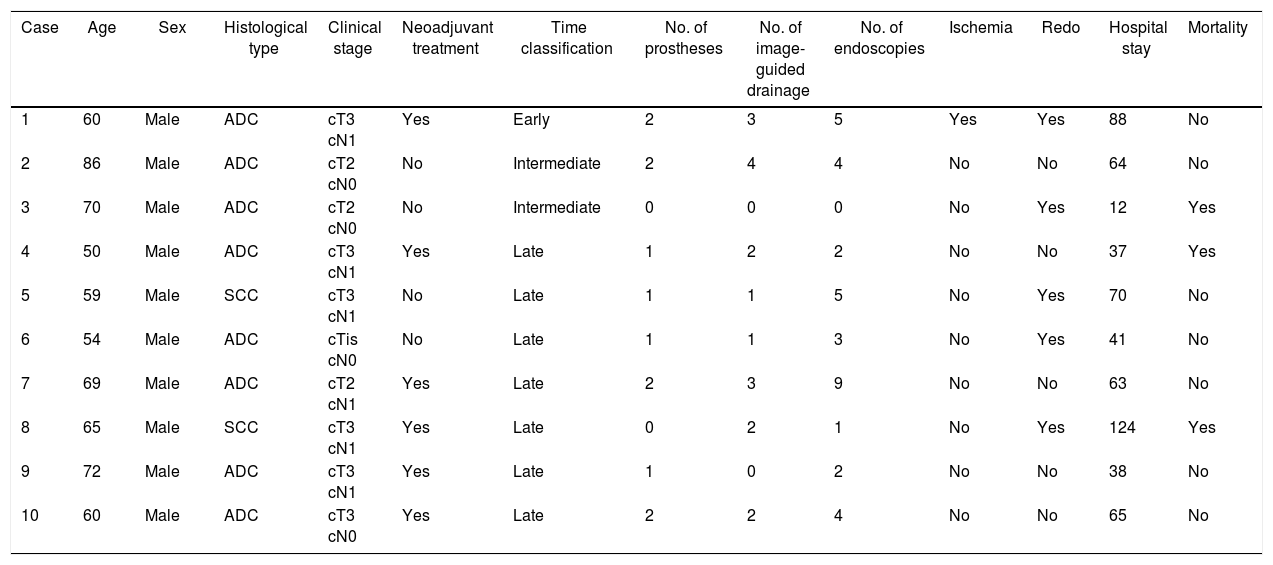
ResultsAnastomotic dehiscence was diagnosed in 10 patients (11.8%), all of them men, with a mean age of 64.5 years (50–86). Epidemiological and tumor stage data for each patient are detailed in Table 1. Regarding the time of the diagnosis of dehiscence, it was late in 7 patients, early in one patient and intermediate in the 2 remaining patients. Three patients of the series died, 2 of whom were re-operated and did not have stents. Of these latter, one presented multiple organ failure associated with persistent bile leak and another developed aspiration pneumonia. Eight patients (80%) had prostheses, and 2 stents were required in 4 patients. Stent migration was observed in one patient (12.5%), and an average of 4.2 endoscopies were performed per patient, mainly to determine the location as well as to rule out possible problems related with the stents, such as decubitus ulcers or inclusion of the esophageal or gastric wall. The average stent use time was 47.3 days. Image-guided drainage was done placed in 7 patients, and 3 required re-operation despite having the stent: one to resolve associated chylothorax, another for pleural and mediastinal debridement, and a third for stent failure, mediastinal lavage and surgical closure of the defect. The mean hospital stay of the patients treated with stents was 61.2 days (38–88), and one patient died out of the 8 (12.5%).
Epidemiological Data, Tumor Stage and Data Related With the Treatment of Patients With Anastomotic Dehiscence After Ivor Lewis Esophagectomy.
| Case | Age | Sex | Histological type | Clinical stage | Neoadjuvant treatment | Time classification | No. of prostheses | No. of image-guided drainage | No. of endoscopies | Ischemia | Redo | Hospital stay | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | Male | ADC | cT3 cN1 | Yes | Early | 2 | 3 | 5 | Yes | Yes | 88 | No |
| 2 | 86 | Male | ADC | cT2 cN0 | No | Intermediate | 2 | 4 | 4 | No | No | 64 | No |
| 3 | 70 | Male | ADC | cT2 cN0 | No | Intermediate | 0 | 0 | 0 | No | Yes | 12 | Yes |
| 4 | 50 | Male | ADC | cT3 cN1 | Yes | Late | 1 | 2 | 2 | No | No | 37 | Yes |
| 5 | 59 | Male | SCC | cT3 cN1 | No | Late | 1 | 1 | 5 | No | Yes | 70 | No |
| 6 | 54 | Male | ADC | cTis cN0 | No | Late | 1 | 1 | 3 | No | Yes | 41 | No |
| 7 | 69 | Male | ADC | cT2 cN1 | Yes | Late | 2 | 3 | 9 | No | No | 63 | No |
| 8 | 65 | Male | SCC | cT3 cN1 | Yes | Late | 0 | 2 | 1 | No | Yes | 124 | Yes |
| 9 | 72 | Male | ADC | cT3 cN1 | Yes | Late | 1 | 0 | 2 | No | No | 38 | No |
| 10 | 60 | Male | ADC | cT3 cN0 | Yes | Late | 2 | 2 | 4 | No | No | 65 | No |
ADC: adenocarcinoma; SCC: squamous-cell carcinoma.
The dehiscence of an intrathoracic esophagogastric anastomosis is one of the most important causes of death after an Ivor Lewis procedure.8 Early diagnosis, the use of antibiotics, antifungal drugs, prokinetic agents and proton pump inhibitors, as well as gastric decompression, are important to reduce this tendency.2 In this context, drainage of the pleural cavity and the mediastinum is used to avoid systemic repercussions. It seems logical to think that, in the absence of massive gastric necrosis, the closure or plugging of the defect is beneficial insofar as it avoids continued contamination. Traditionally, the treatment of dehiscence after Ivor Lewis esophagectomy required a surgical reintervention for hygienization of the surgical field and the control of the defect using several techniques based on the findings. The aggression that represents a new thoracotomy, especially in fragile patients, can paradoxically increase the risk of mortality, as occurred in 2 patients in our series who initially underwent surgery (Table 1).
Self-expanding metal prostheses began to be used successfully in esophageal pathologies as palliative treatment for malignant dysphagia in patients considered inoperable or unresectable disease.9,10 The growth of tumor tissue through the framework of the prosthesis and its inclusion in the esophageal wall, with the consequent perforation that took place in some cases, imposed the need to coat these prostheses with some sort of material that would avoid this problem. The technological development that has taken place in recent decades has meant that today there is a considerable variety of self-expanding prostheses, offering different lengths, shapes and diameters, metallic, partially or totally coated, plastic and even biodegradable. This has allowed for indications of prostheses to be expanded in esophageal pathologies to include the treatment of fistulae after Ivor Lewis procedures. Early dehiscence after transthoracic esophagectomy is usually due to technical defects or acute ischemia of the repair; thus, we are in favor of surgical treatment because, in this situation, the prosthesis was not effective in the patient in our series who presented it. These circumstances may require anywhere from bipolar exclusion to debridement and resuture, with or without a reinforcement flap, or the placement of a T-tube. In our case, we then debrided the mediastinum, excised a small gastric ischemic area and performed a new esophagogastrostomy. For the remaining intermediate and late dehiscences, generally type 2 according to the classification of the Esophagectomy Complications Consensus Group,11 the use of stents has helped accelerate recovery. In this context, Hünerbein et al.12 compared two groups of patients with intrathoracic anastomotic dehiscence: one treated by surgery or conservative treatment and another in which plastic prostheses were used. The authors demonstrated that the use of stents meant that oral intake could be initiated earlier, while decreasing overall hospital stay, Intensive Care Unit stay and mortality. We do not know what would have happened if the prostheses had not been used in our patients, and we cannot compare these results with historical series due to lack of sample size, but there have not been serious complications associated with endoscopic procedures,13 which have been proven safe.14 The only death of a patient with a stent graft in our series was due to pneumonia and renal failure after CT and endoscopy had confirmed correct placement of the prosthesis and the absence of undrained collections.
Despite its advantages, the monitoring of patients with stents must be continuous because the prosthesis can migrate3 or the flares can become loosened, allowing the passage of fluid between the esophageal-gastric walls and the stent, resulting in mediastinal contamination. Some authors, such as Dent et al., 2 argue that self-expandable prostheses should not be part of routine treatment in this type of situation because these procedures provide good results without stent application, using only image-guided drainage or re-operation. These same authors emphasize that the complications derived from the use of stents described in the literature, including some cases of death, are reason enough to limit their use as much as possible. More recently, other authors15 have described the successful use of aspiration therapy in the closure of this type of dehiscence. However, a review of the literature also reveals complications derived from this treatment, such as stenosis.
In conclusion, esophageal stents are safe and effective devices that did not associate mortality in our series. They are especially indicated in intermediate or late dehiscence and in fragile patients, because, when used in conjunction with mediastinal and pleural drainage, they avoid reoperations with their added morbidity and mortality. Therefore, stents should be part of the standard therapeutic arsenal for the resolution of most suture dehiscences after Ivor Lewis esophagectomy. Randomized prospective studies would help determine more precisely the role of these devices in the treatment of dehiscences after transthoracic esophagectomy.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Ruiz de Angulo D, Ortiz MÁ, Munitiz V, Martínez de Haro LF, Alberca F, Serrano A, et al. Papel de las endoprótesis autoexpandibles en el tratamiento de la dehiscencia intratorácica tras el procedimiento de Ivor Lewis. Cir Esp. 2018;96:555–559.