The esophageal cancer surgery is a complex procedure with elevated rates of both morbidity and mortality, which is why, in order to achieve adequate results, it should be performed in high volume centers, where complete multidisciplinary support is available and recent clinical guidelines are applied. We describe the initial experience and the technique of “tubeless” esophagectomy where esophageal resection and mediastinal lymphadenectomy are performed and no drains nor tubes of any kind are placed, with the aim to decrease the level of surgical aggression, enhance the postoperative comfort and accelerate the patient́s recovery.
La cirugía del cáncer de esófago es un procedimiento complejo con tasas de morbimortalidad elevadas, por lo que para obtener resultados adecuados se precisa de centros experimentados, un completo soporte multidisciplinar y vías clínicas adecuadas. Se describe la experiencia inicial y la técnica de la esofaguectomía «tubeless» en la que tras realizar una resección esofágica y linfadenectomía mediastínica extendida, al final del procedimiento no son colocados drenajes ni sondas de ningún tipo, con el fin de disminuir la agresividad del mismo, mejorar el bienestar postoperatorio y acelerar la recuperación funcional del paciente.
Esophageal cancer surgery is a complex procedure with morbidity rates of 50%–60% and postoperative mortality from 2% to 5%.1 Currently, with the development of minimally invasive surgery, the preoperative optimization of the nutritional, physical and psychological state of the patient, improvements in anesthetic management and pain control, and the protocolized application of other multimodal rehabilitation measures, satisfactory results are obtained. This facilitates recovery and reduces hospital stay as well as certain postoperative complications.2
In 2019, Low et al. published the Guidelines for Perioperative Care in Esophagectomy, which included the measures recommended by the ERAS Society3 for patients undergoing resection for esophageal cancer. In the perioperative stage, these guidelines recommend the use of a nasogastric tube, chest drain tubes and early enteral feeding in the first 3–6 postoperative days in order to meet nutritional requirements, either by jejunostomy or a nasojejunal or nasoduodenal tube.
However, to achieve a faster, total postoperative functional recovery, progress is being made in reducing surgical aggression and the use of these elements. This has raised questions about certain measures whose use is not supported by solid evidence, such as drain tubes, nasogastric tubes or feeding jejunostomy.
The following is a description of the technique, perioperative management, and our initial experience with the application of ‘tubeless’ esophagectomy, using a minimally invasive approach and without the use of any type of drain tube, nasogastric tube or enteral feeding tube in patients with esophageal cancer.
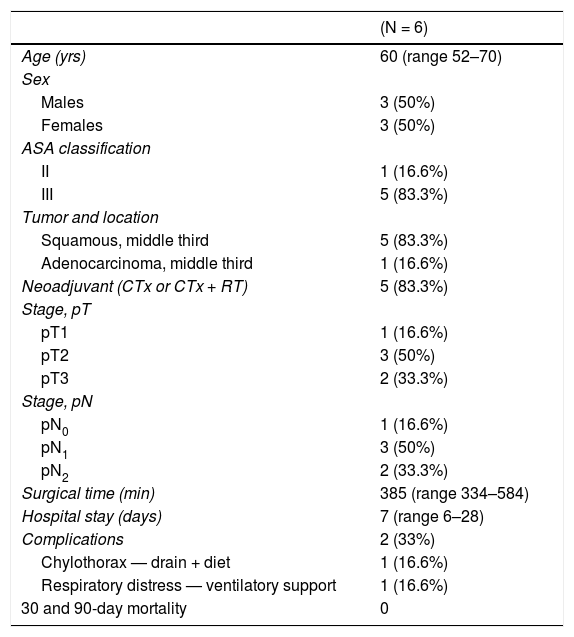
Surgical technique and perioperative management. ResultsFrom June to November 2020, a total of 6 patients (3 men and 3 women) were treated with this technique and perioperative management at our hospital. Median age was 60 years (range: 52–70) (Table 1), and the majority (83.3%) had squamous cell carcinoma located in the middle third of the esophagus. Four patients had received neoadjuvant treatment in accordance with the CROSS scheme.4
Descriptive study of cases.
| (N = 6) | |
|---|---|
| Age (yrs) | 60 (range 52–70) |
| Sex | |
| Males | 3 (50%) |
| Females | 3 (50%) |
| ASA classification | |
| II | 1 (16.6%) |
| III | 5 (83.3%) |
| Tumor and location | |
| Squamous, middle third | 5 (83.3%) |
| Adenocarcinoma, middle third | 1 (16.6%) |
| Neoadjuvant (CTx or CTx + RT) | 5 (83.3%) |
| Stage, pT | |
| pT1 | 1 (16.6%) |
| pT2 | 3 (50%) |
| pT3 | 2 (33.3%) |
| Stage, pN | |
| pN0 | 1 (16.6%) |
| pN1 | 3 (50%) |
| pN2 | 2 (33.3%) |
| Surgical time (min) | 385 (range 334–584) |
| Hospital stay (days) | 7 (range 6–28) |
| Complications | 2 (33%) |
| Chylothorax — drain + diet | 1 (16.6%) |
| Respiratory distress — ventilatory support | 1 (16.6%) |
| 30 and 90-day mortality | 0 |
Approximately 4–6 weeks before the operation, a preoperative functional optimization protocol was applied. Three weeks before, the gastric plasty was conditioned by embolization of the splenic and left gastric arteries using Interlock™ coils (Boston Scientific, Voisins-le-Bretonneux, France).
All patients underwent 3-stage esophagectomy with a minimally invasive approach (right thoracoscopy in the prone position, laparoscopy, and left cervicotomy).
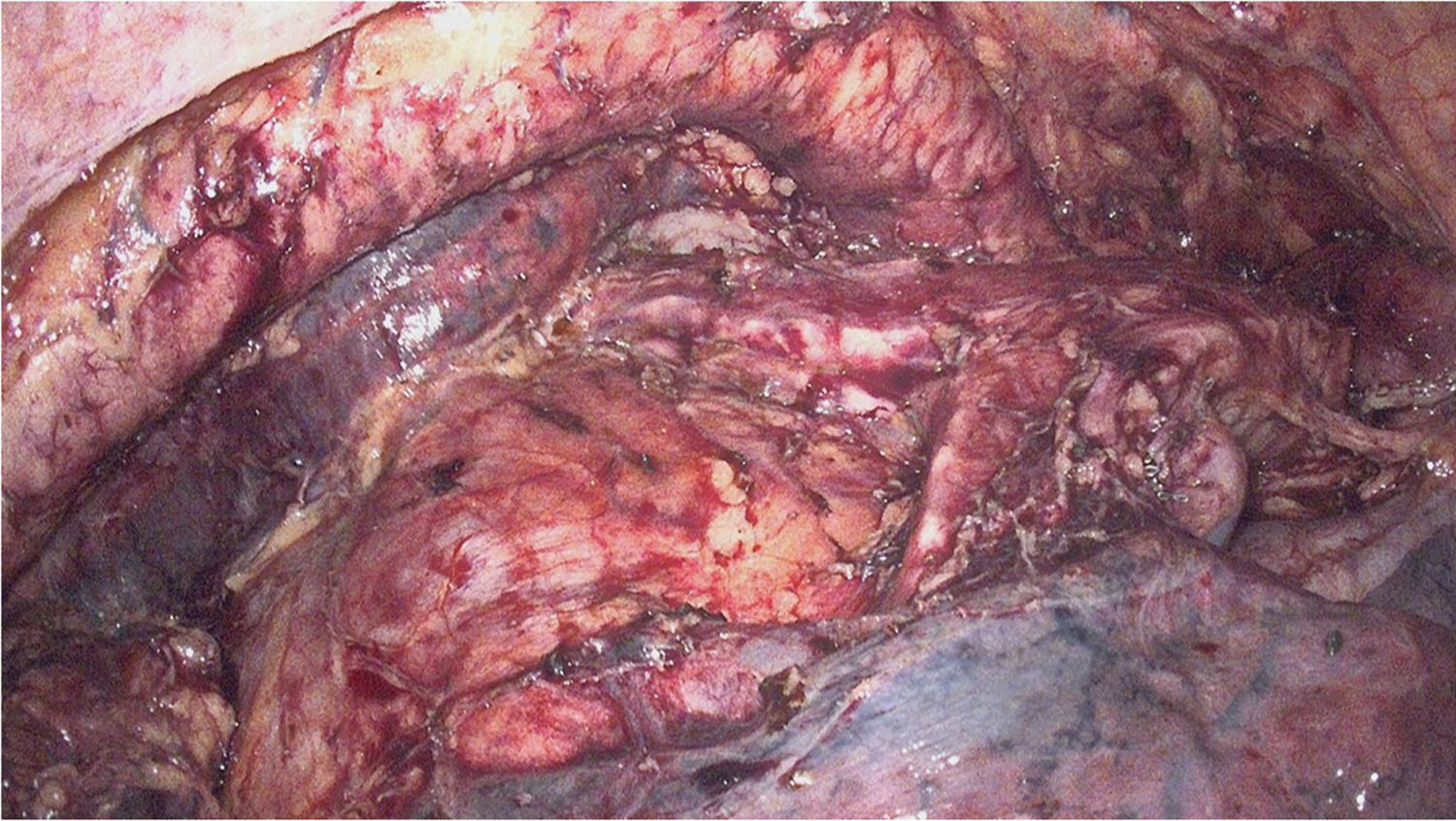
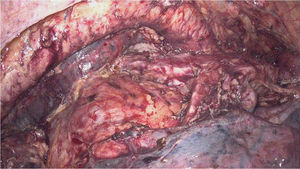
During right thoracoscopy, an incision was made in the mediastinal pleura, mobilizing the esophagus en bloc along with its mesentery. After dissection and division of the azygos vein at its arch, total mediastinal lymphadenectomy was performed including the mesoesophagus, thoracic duct, both mediastinal pleurae and lymph node groups 105, 106, 107, 108, 109, 110, 111 and 122, in accordance with the Japanese Classification of Esophageal Cancer. Both recurrent nerves were identified and spared, and the vagus nerves were divided distally to the bronchial branches (Fig. 1).
The abdominal approach was performed with the patient in the supine position. We completed the oncological resection (lymph node dissection of lymph node groups 1, 2, 3, 7, 8, 9, 11, 19 and 20, according to the Japanese Classification of Esophageal Cancer), while preserving the right gastroepiploic vessels and creating the gastric conduit with the help of the Echelon Flex™ (Johnson and Johnson) or Signia™ (Medtronic) stapling system.
The cervical esophagus was then dissected and divided using a left lateral cervicotomy. At the cranial end of the divided esophagus, a tobacco pouch was then created with 2/0 Prolene® (Johnson and Johnson) and a nasogastric tube was attached to its caudal end. After performing a midline laparotomy of about 5 cm, the surgical specimen attached to the probe was extracted and then the gastroplasty was pulled up transmediastinally. Once the conduit was positioned in its final place, intravenous indocyanine green was administered and correct perfusion of the future area of the anastomosis was verified, which was created at the cervical level in a mechanical end-to-side manner with 25 mm CEEA and then covered with an omental patch.
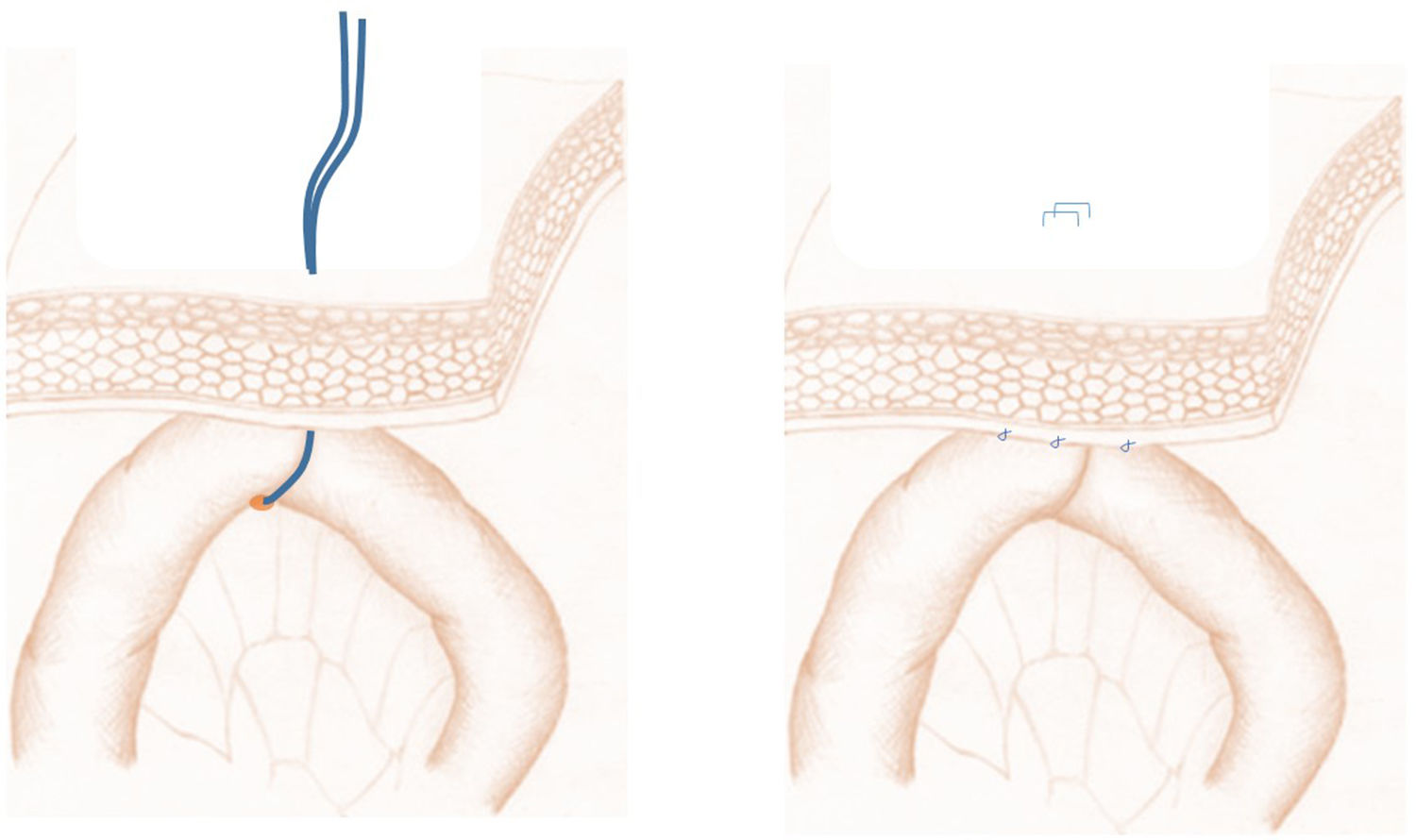
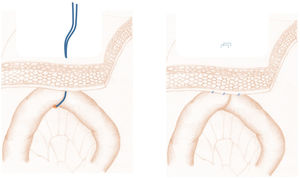
Next, we performed the so-called ‘phantom’ jejunostomy (Fig. 2), which is a new concept that consists of marking the first jejunal loop with a transcutaneous vessel loop or affixing it to the parietal peritoneum, then marking this point of fixation on the skin. During the postoperative period, if necessary, a 12 Fr catheter (Mac Loc® Locking multipurpose drainage catheter from Cook Medical) could be placed under ultrasound guidance percutaneously inside said loop, which would be used as a feeding jejunostomy. When this was not necessary, the vessel loop was removed 10 days after the procedure.
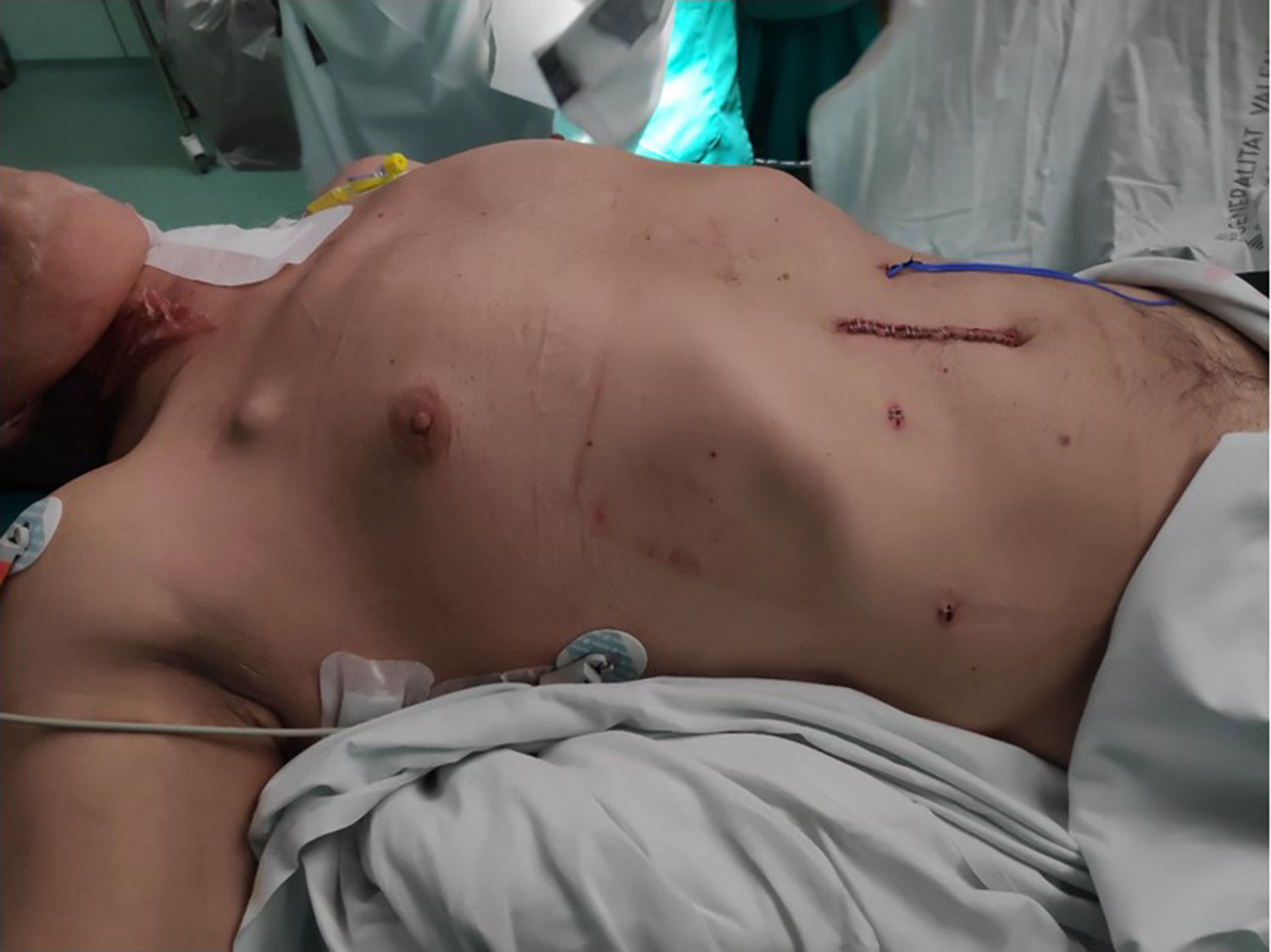
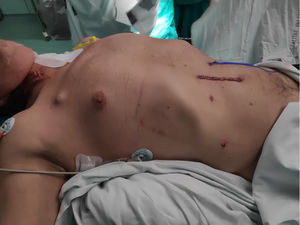
Once the reconstructive phase had been completed, if there were no alterations in hemostasis, injury to the lung parenchyma, or other intraoperative complications, no nasogastric tube or any type of drain tube was used (Fig. 3).
All patients were extubated at the end of the surgery, remaining in the Resuscitation Unit with ventilatory support using high-flow nasal cannula for the first 24–48 h. The urinary and epidural catheters were usually removed 24–48 h after surgery, respectively, while sitting and oral fluid tolerance was initiated in the first 24–48 h. The consistency of the diet and the degree of mobilization were increased in subsequent days.
During the postoperative period, patients were monitored by a multidisciplinary team of specialists (endocrinologists, nutritionists, respiratory physiotherapist, anesthetist, surgeons), with a median hospital stay of 7 days (range: 6–28). There were no anastomotic complications, nor was it necessary to place a jejunostomy, nasogastric tube, or reoperate any patient. One patient developed chylothorax on the fifth postoperative day, which required the placement of a pleural drain; the condition was resolved with dietary measures, and the drain was removed 8 days later. Another patient with a history of pulmonary emphysema developed acute respiratory distress and pneumothorax on the sixth postoperative day, requiring placement of a left chest drain and respiratory support in the Resuscitation Unit. There was no mortality 30 and 90 days after the procedure (Table 1).
After the pathological study of the resected pieces, 50% of T2 tumors were reported; tumor invasion was isolated in 83.3% of lymph nodes (Table 1). The proximal, distal and radial margins showed no tumor involvement in any case.
DiscussionThe technique and perioperative care measures described and included under the concept of ‘tubeless’ esophagectomy are feasible and, in selected cases, could improve and accelerate the postoperative recovery of patients undergoing this procedure. This type of management has not been previously described in the literature, including measures that reduce surgical aggression, pain and discomfort experienced by these patients due to the placement of tubes and drains.
This surgical procedure entails high postoperative morbidity and mortality rates, which is why a multidisciplinary approach is necessary at experienced medical centers, where effective and rapid treatment can also be guaranteed in the event of potential postoperative complications. In this context, the centralization of these procedures5 and the development and application of perioperative medicine protocols (to optimize the patient’s condition in order to face surgery in ideal conditions and accelerate their subsequent recovery) have been shown to help reduce the potential complications of this technique.6,7 Although protocols and multimodal rehabilitation pathways for esophagectomy have already been published,3,8 many aspects are still controversial.
Thus, the preferred feeding method during the postoperative period after esophagectomy is currently the subject of discussion. Recent studies have shown that early oral intake seems safe and is associated with quicker recovery of the gastrointestinal transit and shorter hospital stays,9–11 as described in the patients presented with fluid intake in the first 24–48 h and who showed good oral tolerance to a puréed diet 4–5 days after the procedure in most cases.
‘Phantom’ jejunostomyThe use of enteral feeding tubes (jejunostomy, nasojejunal, etc.) is effective and very useful in situations that require prolonged fasting, when minimum nutritional requirements cannot be met orally, or in patients with aphagia and malnutrition during the perioperative stage. However, their systematic placement is not without complications,12 while they also increase patient discomfort and limit free movement, so their use must be considered individually. In this sense, the novel concept of the ‘phantom’ jejunostomy aims to avoid routine placement and potential associated complications, while facilitating rapid access for the introduction of an enteral feeding tube through a local ultrasound-guided approach in cases where it is indicated.
No use of nasogastric tubesTraditionally, the use of a nasogastric tube after esophagectomy has been considered mandatory and still continues to be recommended by multiple guidelines3 in order to decompress the reconstructive plasty, avoid its dilation, reduce anastomotic tension and avoid vomiting, pain and possible aspirations. However, there are contradictory data in the literature regarding its use and the risk of anastomotic and respiratory complications. In our experience, the use of nasogastric tubes has not shown benefits in reducing complications after esophagectomy; however, it has shown a delay in the onset of oral tolerance, which lengthens the hospital stay.13 Likewise, other studies state that the immediate or early removal of the nasogastric tube does not increase the number of anastomotic dehiscences, pulmonary complications or postoperative mortality, thereby reducing the hospital stay and patient discomfort, while accelerating oral tolerance.14,15
No systematic use of drainsThe use of a cervical drain after esophagectomy has not been shown to reduce the number of local wound complications, such as hematoma, seroma or anastomotic dehiscence,16 and therefore its systematic use is not recommended.
The currently available evidence to support the use of chest drains after esophagectomy is very limited, although most published guidelines include it in their recommendations3 since it could prevent pulmonary compression and be used to monitor the presence of bleeding and/or leaks (air, chylous, or anastomotic). However, their use causes greater pain, which results in worse ventilation and patient mobility.17 Some published series have shown that the use of a single drain is effective and reduces postoperative pain, costs and hospital stay compared to placing a greater number of them.18–20 In this same context, their early removal (at a discharge lower than 400 mL/24 h with no air, anastomotic or chylous leaks) is safe and could improve postoperative well-being and reduce hospital stay;21–23 recently published case series of major lung resections have shown that the non-use of thoracic drains is feasible and safe.24
In our experience, patients who are candidates for not placing a chest drain tube are cooperative, trained in respiratory physiotherapy programs, have adequate physical condition and lung function, and undergo esophagectomy with no intraoperative complications. Obviously, it is mandatory to have the ability to drain liquid or air collections immediately and efficiently if necessary, such as in the cases of pneumo- and chylothorax presented in this series.
Regarding the use of abdominal drains, randomized studies and reviews of published evidence25–27 have established that their use after gastrectomy does not offer benefits. Therefore, their use after esophagectomy is not recommended by several published guidelines.3,8
To conclude, tubeless esophagectomy is a feasible concept that can improve postoperative recovery in selected cases, reducing the pain associated with drain and feeding tubes that are usually inserted, facilitating early mobilization and correct performance of respiratory physiotherapy exercises, and improving functional recovery and quality of life during the postoperative period of this surgery. Well-designed studies with a greater number of cases are necessary to solidly assess the pros and cons of this type of procedure.
Conflict of interestsThe authors have no conflict of interests to declare regarding this manuscript.
Please cite this article as: Bruna M, Mingol F, Navasquillo M, Cholewa H, Vaqué FJ. Esofaguectomía «tubeless»: menos es más. Cir Esp. 2021;99:457–462.