Nasogastric decompressive tube utilization has been accepted as one of the basic perioperative care measures after esophageal resection surgery. However, with the development of multimodal rehabilitation programs and without clear evidence to support their use, the systematic indication of this measure may be controversial.
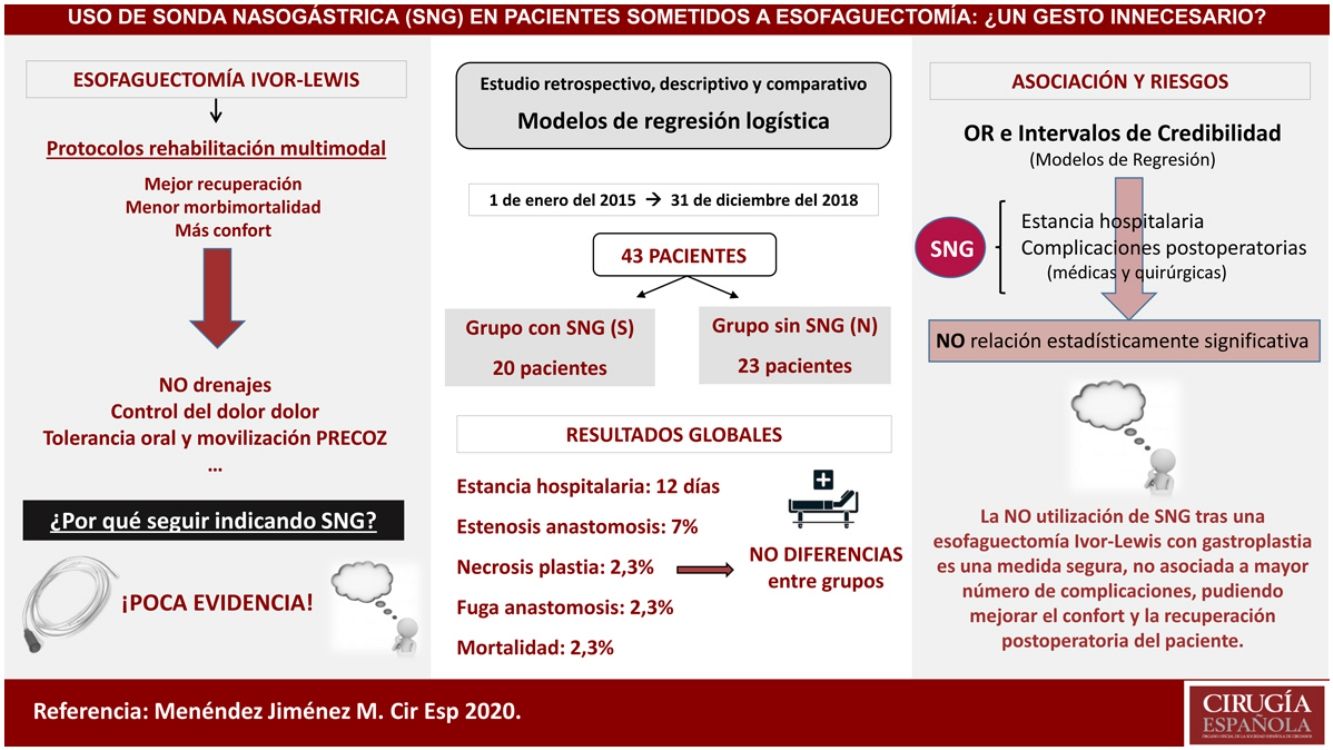
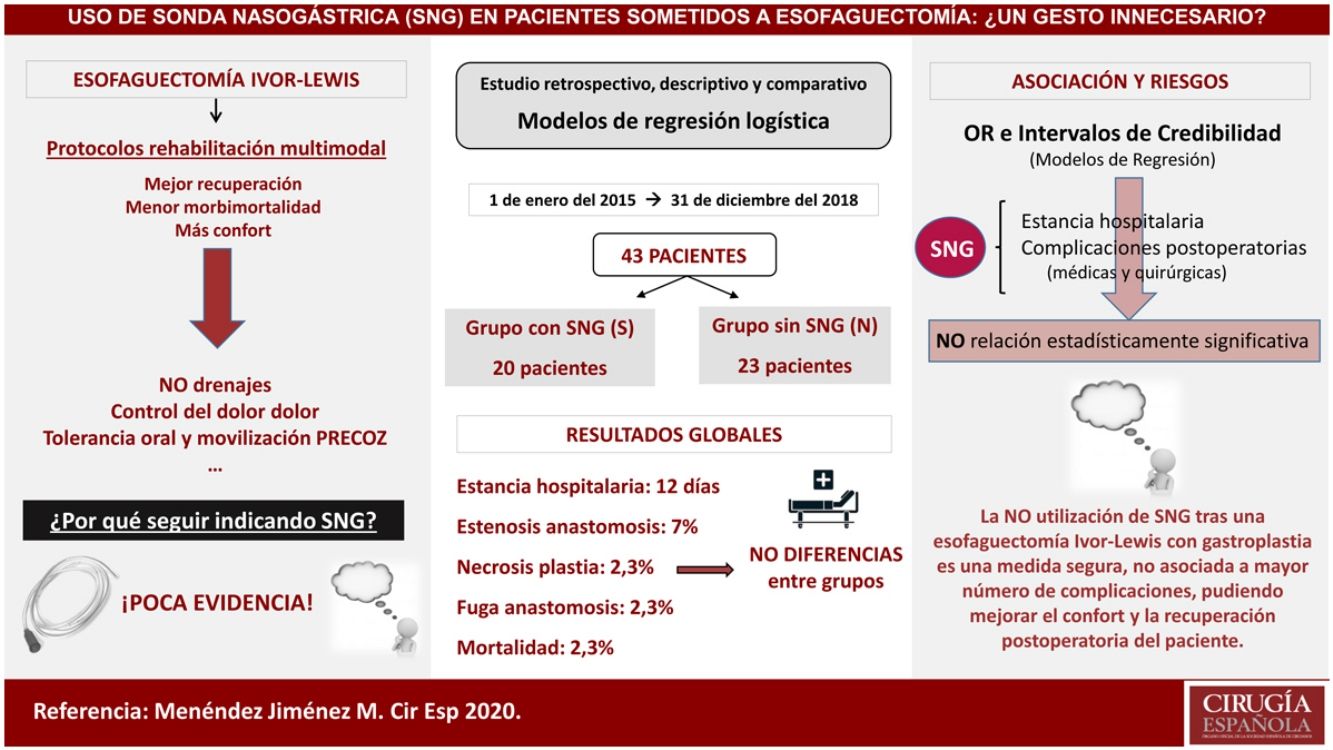
Material and methodsRetrospective, descriptive and comparative study of patients who had undergone Ivor-Lewis esophagectomy in our center – from January 2015 to December 2018 – with placement (Group S), or without placement (Group N) of a decompressive tube in gastroplasty during postoperative period. Epidemiological variables and differences between groups in post-surgical morbidity and mortality, hospital stay, onset of oral tolerance and the need for nasogastric tube placement were evaluated.
ResultsA total of 43 patients were included in this study, with a median age of 61 years, being 86% male. 46.5% were hypertensive, 25.5% had lung disease and 16.3% had diabetes mellitus. The median length of hospital stay was 9 days in group S versus 11.5 days in group N, with no differences in the onset of oral tolerance. Anastomotic dehiscence rate was 5% and 0% respectively. The overall mortality was 2.3% in the first 90 days, without differences between the groups. Placement of nasogastric tube during postoperative period was required only in 1 patient (4.3%) of the group N.
ConclusionsNon-use of nasogastric tube during postoperative period of an Ivor-Lewis esophagectomy is a safe measure, as it is not associated with a higher rate of complications or hospital stay. This fact may be able to improve patients’ comfort and postoperative recovery.
El empleo de una sonda descompresiva nasogástrica es aceptado como uno de los cuidados perioperatorios básicos tras una cirugía de resección esofágica. Sin embargo, con el desarrollo de los programas de rehabilitación multimodal en este campo y sin una evidencia clara que sustente su empleo, la indicación sistemática de dicha medida puede resultar controvertida.
Material y métodosEstudio retrospectivo, descriptivo y comparativo de los casos intervenidos de esofaguectomía tipo Ivor-Lewis en nuestro centro desde enero de 2015 hasta diciembre de 2018 con colocación (Grupo S) o no de sonda (Grupo N) descompresiva en la plastia gástrica durante el postoperatorio. Se evaluaron variables epidemiológicas y diferencias entre los grupos en morbimortalidad postquirúrgica, estancia hospitalaria, inicio de la tolerancia oral y la necesidad de colocación de sonda nasogástrica.
ResultadosUn total de 43 pacientes fueron incluidos en este estudio con una mediana de edad de 61 años, siendo el 86% varones. El 46,5% eran hipertensos, el 25,5% presentaban enfermedad pulmonar y el 16,3% padecían diabetes mellitus. La mediana del tiempo de estancia hospitalaria fue de nueve días en el grupo S frente a 11,5 días del grupo N, sin diferencias en el inicio de la tolerancia oral. La tasa de dehiscencia anastomótica fue del 5% y del 0%, respectivamente. La mortalidad global fue del 2,3% en los primeros 90 días, sin diferencias entre los grupos y la necesidad de colocación de la sonda durante el postoperatorio se produjo únicamente en un paciente (4,3%) del grupo N.
ConclusionesLa no utilización de sonda nasogástrica durante el postoperatorio de una esofaguectomía tipo Ivor-Lewis es una medida segura y no está asociada a mayor número de complicaciones ni estancia hospitalaria, pudiendo mejorar la comodidad y la recuperación postoperatoria del paciente.
Esophagectomy is the procedure of choice for the treatment of patients with malignant tumors that meet criteria for resectability and operability. It is also used to treat certain benign pathologies that require it due to their location or clinical situation. Many perioperative measures are applied to try to increase the safety of this procedure and reduce the high morbidity and mortality associated with it.
With the development of multimodal rehabilitation protocols, some of the traditionally applied measures have been reassessed, and their use has been modified based on the most current scientific evidence. Thus, the optimization of the patient's nutritional, psychological and physical state, together with the correction of anemia, are basic points for the preparation of these patients. Similarly, the initiation of oral intake and early mobilization during the postoperative period are part of this type of program, as well as the use of drain systems, which usually seem to be restricted to tubes placed in the pleural cavity, at least for the moment.1,2 The latest consensus documents and clinical guidelines published by different societies recommend the routine use of a nasogastric tube (NGT), which has created controversy because current evidence supporting its use is still limited.1,2
The objective of this study is to evaluate and compare the results obtained in patients treated with Ivor-Lewis esophagectomy based on the use or not of a nasogastric tube for decompression during the immediate postoperative period.
MethodsWe designed a retrospective, descriptive and comparative study of all patients who underwent esophagectomy between January 2015 and December 2018 at our hospital. The study included all patients diagnosed with malignant esophageal neoplasms or complicated benign disease who underwent elective surgery with Ivor-Lewis esophagectomy using an abdominal and transthoracic approach, reconstruction with gastroplasty and no associated pyloroplasty. Patients requiring urgent surgery were excluded. The series was divided into two consecutive groups: group S, which included patients in whom a nasogastric tube was placed in the gastroplasty for decompression during the immediate postoperative period (from January 2015 to October 2016); and group N, in which tubes were not inserted or used during the perioperative period (from November 2016 to December 2018). The interventions were performed in the same hospital by two surgeons with extensive experience in esophageal surgery.
In the preoperative study, in addition to the diagnosis for which esophagectomy was indicated, epidemiological variables and different comorbidities were collected, such as age, sex, personal history or previous surgeries, American Society of Anesthesiologists (ASA) classification, tumor type and stage, and neoadjuvant treatment.
All patients underwent gastroplasty conditioning three weeks before surgery by embolization of the splenic and left gastric arteries. Similarly, all patients received mechanical preparation of the colon using sodium picosulfate in the 24h prior to surgery, in addition to the same antibiotic prophylaxis regimen, antithromboembolic measures (low-molecular-weight heparin and compression stockings) and prophylaxis for perioperative nausea and vomiting. In all patients, either one or two chest drain tubes with suction were inserted. Abdominal drain tubes were not placed, nor was any technique performed on the pylorus to facilitate emptying of the plasty in any of the patients. As of January 2018, the performance of a jejunostomy in the same operation was limited to more selected cases with malnutrition or a higher risk of nutritional problems, based on the results obtained in our unit.3
We evaluated the surgical approach, surgical time and the creation or not of a jejunostomy.
During the postoperative period, all patients underwent a radiological study with oral contrast. We also analyzed the presence of nausea or vomiting, time until oral and/or enteral tolerance (in cases of jejunostomy), need for of nasogastric tube placement for decompression, and the length of stay in the hospital and in the resuscitation unit. Postoperative complications, mortality in the first 90 days and the readmission rate were also collected.
The variables and results have been described as median and interquartile range (IQR) in the case of continuous variables and as frequencies in the case of categorical variables.
To determine the possible association between the use of NGT and the various types of complications, different logistic regression models have been adjusted. The use of NGT was the main predictor variable. Sex, body mass index (BMI), anesthetic risk according to the ASA scale, diabetes mellitus, chronic renal failure, tobacco habit or lung disease, and the minimally invasive approach were included as covariates as they were possible confounding factors.
Given the high number of variables and the small effective sample size in most cases, the different models have been adjusted using Bayesian statistics, applying horseshoe priors to the model coefficients to avoid overfitting.3 In the case of days of hospital stay (continuous variable), a linear regression model was developed. On all adjusted models, 95% credible intervals were estimated. In the event that a credible interval for an odds ratio (OR) did not include 1, the effect of the corresponding variable was considered significant on the response variable.4
ResultsA total of 43 patients were treated consecutively with Ivor-Lewis esophagectomy and gastroplasty at our hospital during the study period (January 2015 to December 2018): 20 in group S, and 23 in group N.
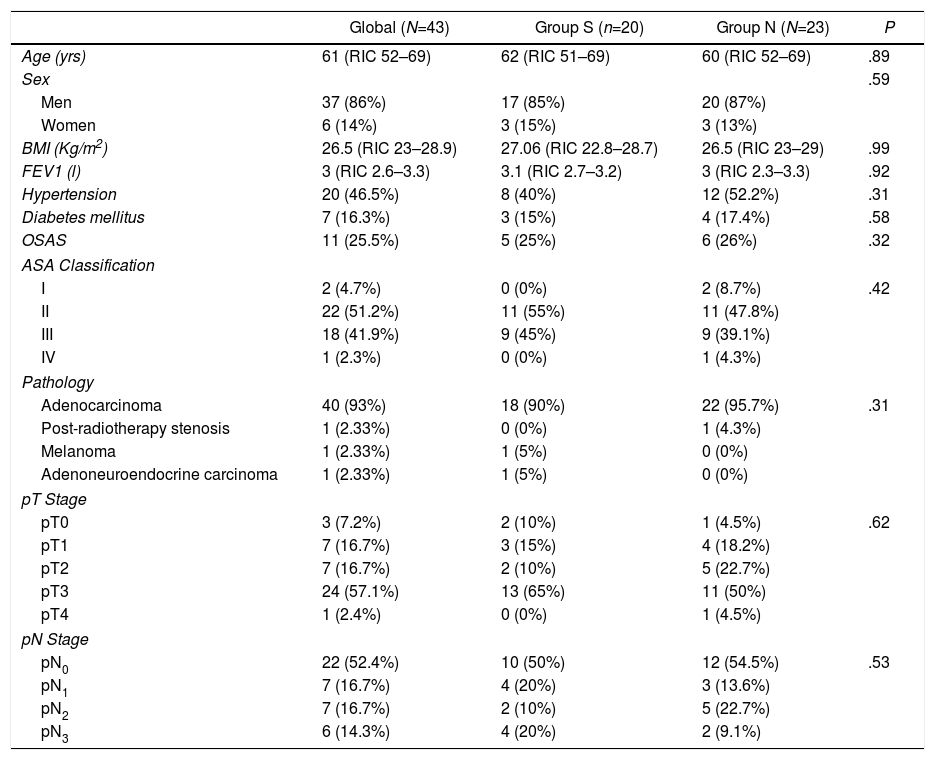
86% of patients were men, and the median age of the group was 61 years (IQR: 52–69). Table 1 shows the demographic characteristics of the patients, and there were no significant differences between the two groups in age, sex or ASA classification (most were classified as ASA II–III). The etiologies for which the surgery was indicated were mostly of tumor origin (97.6%). 60.4% of the patients received neoadjuvant treatment with chemotherapy and 4.7% treatment with chemotherapy plus neoadjuvant radiotherapy (Table 1).
Descriptive Study of patient characteristics.
| Global (N=43) | Group S (n=20) | Group N (N=23) | P | |
|---|---|---|---|---|
| Age (yrs) | 61 (RIC 52–69) | 62 (RIC 51–69) | 60 (RIC 52–69) | .89 |
| Sex | .59 | |||
| Men | 37 (86%) | 17 (85%) | 20 (87%) | |
| Women | 6 (14%) | 3 (15%) | 3 (13%) | |
| BMI (Kg/m2) | 26.5 (RIC 23–28.9) | 27.06 (RIC 22.8–28.7) | 26.5 (RIC 23–29) | .99 |
| FEV1 (l) | 3 (RIC 2.6–3.3) | 3.1 (RIC 2.7–3.2) | 3 (RIC 2.3–3.3) | .92 |
| Hypertension | 20 (46.5%) | 8 (40%) | 12 (52.2%) | .31 |
| Diabetes mellitus | 7 (16.3%) | 3 (15%) | 4 (17.4%) | .58 |
| OSAS | 11 (25.5%) | 5 (25%) | 6 (26%) | .32 |
| ASA Classification | ||||
| I | 2 (4.7%) | 0 (0%) | 2 (8.7%) | .42 |
| II | 22 (51.2%) | 11 (55%) | 11 (47.8%) | |
| III | 18 (41.9%) | 9 (45%) | 9 (39.1%) | |
| IV | 1 (2.3%) | 0 (0%) | 1 (4.3%) | |
| Pathology | ||||
| Adenocarcinoma | 40 (93%) | 18 (90%) | 22 (95.7%) | .31 |
| Post-radiotherapy stenosis | 1 (2.33%) | 0 (0%) | 1 (4.3%) | |
| Melanoma | 1 (2.33%) | 1 (5%) | 0 (0%) | |
| Adenoneuroendocrine carcinoma | 1 (2.33%) | 1 (5%) | 0 (0%) | |
| pT Stage | ||||
| pT0 | 3 (7.2%) | 2 (10%) | 1 (4.5%) | .62 |
| pT1 | 7 (16.7%) | 3 (15%) | 4 (18.2%) | |
| pT2 | 7 (16.7%) | 2 (10%) | 5 (22.7%) | |
| pT3 | 24 (57.1%) | 13 (65%) | 11 (50%) | |
| pT4 | 1 (2.4%) | 0 (0%) | 1 (4.5%) | |
| pN Stage | ||||
| pN0 | 22 (52.4%) | 10 (50%) | 12 (54.5%) | .53 |
| pN1 | 7 (16.7%) | 4 (20%) | 3 (13.6%) | |
| pN2 | 7 (16.7%) | 2 (10%) | 5 (22.7%) | |
| pN3 | 6 (14.3%) | 4 (20%) | 2 (9.1%) | |
BMI: body mass index; FEV1: forced expiratory volume in 1 second; OSAS: obstructive sleep apnea syndrome; ASA: American Society of Anesthesiologists.
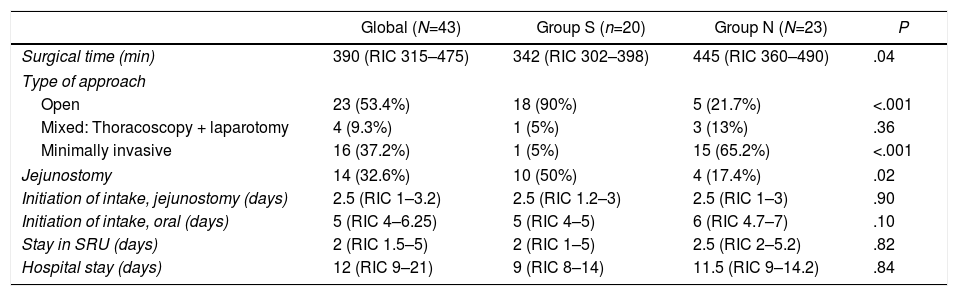
An open approach was used in 53.4% of the patients, with a higher percentage in group S, while a minimally invasive approach was used in 37.2%, more frequently in group N (Table 2). The surgical time was longer in this latter group. The number of jejunostomies performed was higher in the group with NGT, according to the change in approach in the use of this technique that occurred in our unit in January 2018. Initiation of enteral nutrition by jejunostomy occurred in the first 48–72h after surgery in most patients. The median oral intake time was five days after surgery, with no significant differences between the groups. In group S, the median time that patients had the NGT in place was four days (IQR: 2–6).
Characteristics of the surgery and postoperative course.
| Global (N=43) | Group S (n=20) | Group N (N=23) | P | |
|---|---|---|---|---|
| Surgical time (min) | 390 (RIC 315–475) | 342 (RIC 302–398) | 445 (RIC 360–490) | .04 |
| Type of approach | ||||
| Open | 23 (53.4%) | 18 (90%) | 5 (21.7%) | <.001 |
| Mixed: Thoracoscopy + laparotomy | 4 (9.3%) | 1 (5%) | 3 (13%) | .36 |
| Minimally invasive | 16 (37.2%) | 1 (5%) | 15 (65.2%) | <.001 |
| Jejunostomy | 14 (32.6%) | 10 (50%) | 4 (17.4%) | .02 |
| Initiation of intake, jejunostomy (days) | 2.5 (RIC 1–3.2) | 2.5 (RIC 1.2–3) | 2.5 (RIC 1–3) | .90 |
| Initiation of intake, oral (days) | 5 (RIC 4–6.25) | 5 (RIC 4–5) | 6 (RIC 4.7–7) | .10 |
| Stay in SRU (days) | 2 (RIC 1.5–5) | 2 (RIC 1–5) | 2.5 (RIC 2–5.2) | .82 |
| Hospital stay (days) | 12 (RIC 9–21) | 9 (RIC 8–14) | 11.5 (RIC 9–14.2) | .84 |
SRU: Surgery Recovery Unit.
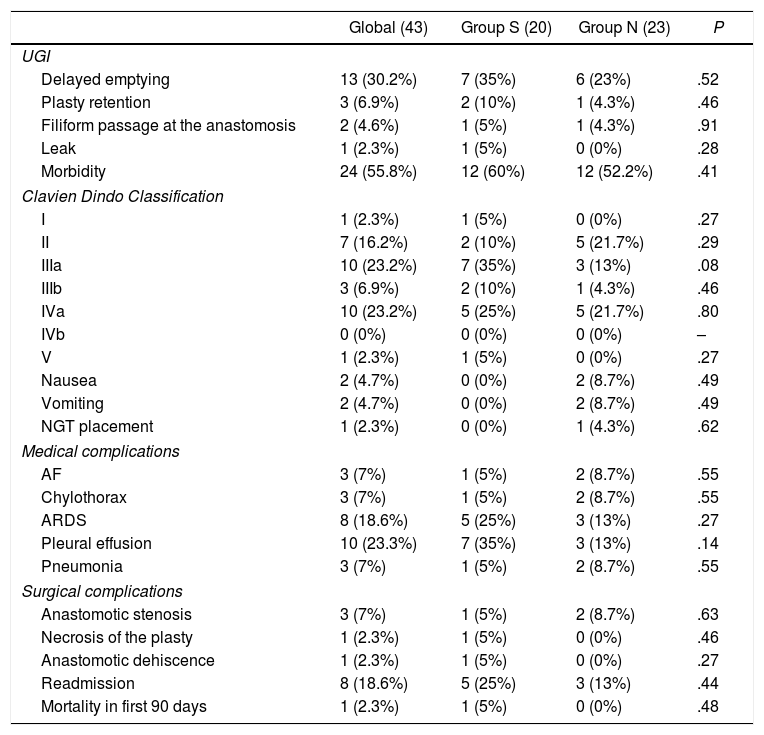
Some type of alteration was observed in the upper gastrointestinal series of 34.9% of patients: 40% in group S, and 30.4% in group N. Delayed emptying of the plasty was seen in 30.2%, and two patients presented difficult passage of the contrast through the anastomosis, with no differences observed between groups (Table 3). None of the patients presented either passage of contrast to the airway or uncoordinated swallowing.
Postoperative morbidity, mortality and rate of readmission.
| Global (43) | Group S (20) | Group N (23) | P | |
|---|---|---|---|---|
| UGI | ||||
| Delayed emptying | 13 (30.2%) | 7 (35%) | 6 (23%) | .52 |
| Plasty retention | 3 (6.9%) | 2 (10%) | 1 (4.3%) | .46 |
| Filiform passage at the anastomosis | 2 (4.6%) | 1 (5%) | 1 (4.3%) | .91 |
| Leak | 1 (2.3%) | 1 (5%) | 0 (0%) | .28 |
| Morbidity | 24 (55.8%) | 12 (60%) | 12 (52.2%) | .41 |
| Clavien Dindo Classification | ||||
| I | 1 (2.3%) | 1 (5%) | 0 (0%) | .27 |
| II | 7 (16.2%) | 2 (10%) | 5 (21.7%) | .29 |
| IIIa | 10 (23.2%) | 7 (35%) | 3 (13%) | .08 |
| IIIb | 3 (6.9%) | 2 (10%) | 1 (4.3%) | .46 |
| IVa | 10 (23.2%) | 5 (25%) | 5 (21.7%) | .80 |
| IVb | 0 (0%) | 0 (0%) | 0 (0%) | – |
| V | 1 (2.3%) | 1 (5%) | 0 (0%) | .27 |
| Nausea | 2 (4.7%) | 0 (0%) | 2 (8.7%) | .49 |
| Vomiting | 2 (4.7%) | 0 (0%) | 2 (8.7%) | .49 |
| NGT placement | 1 (2.3%) | 0 (0%) | 1 (4.3%) | .62 |
| Medical complications | ||||
| AF | 3 (7%) | 1 (5%) | 2 (8.7%) | .55 |
| Chylothorax | 3 (7%) | 1 (5%) | 2 (8.7%) | .55 |
| ARDS | 8 (18.6%) | 5 (25%) | 3 (13%) | .27 |
| Pleural effusion | 10 (23.3%) | 7 (35%) | 3 (13%) | .14 |
| Pneumonia | 3 (7%) | 1 (5%) | 2 (8.7%) | .55 |
| Surgical complications | ||||
| Anastomotic stenosis | 3 (7%) | 1 (5%) | 2 (8.7%) | .63 |
| Necrosis of the plasty | 1 (2.3%) | 1 (5%) | 0 (0%) | .46 |
| Anastomotic dehiscence | 1 (2.3%) | 1 (5%) | 0 (0%) | .27 |
| Readmission | 8 (18.6%) | 5 (25%) | 3 (13%) | .44 |
| Mortality in first 90 days | 1 (2.3%) | 1 (5%) | 0 (0%) | .48 |
UGI: upper gastrointestinal series; NGT: nasogastric tube; AF: atrial fibrillation; ARDS: acute respiratory distress syndrome.
Some type of postoperative complication occurred in 60% of group S and in 52.2% of group N (Table 3). There were no differences in the number of postoperative complications, hospital stay or 90-day mortality between the groups. Only one patient from group N required NGT placement during the postoperative period due to paralytic ileus.
An anastomotic leak was observed in one patient in group S (Table 3), requiring reoperation for treatment.
The overall readmission rate was 18.6%; readmission was more frequent in the catheter group, although the differences were not significant. The 90-day mortality rates were 5% in the NGT group and 0% in the group without a catheter.
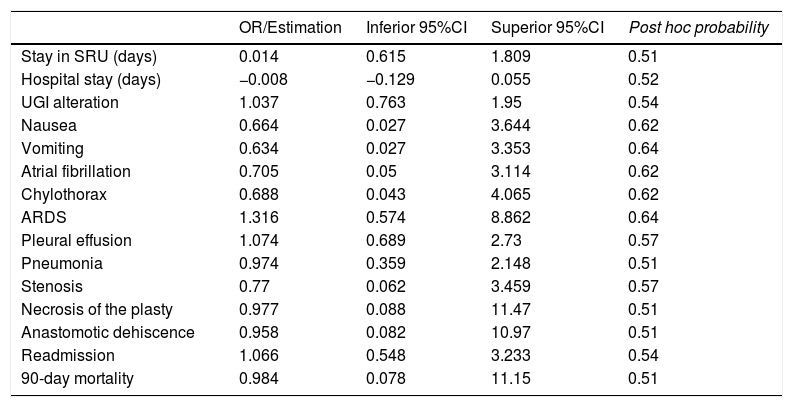
The OR and credible intervals obtained for each variable in the regression models created did not show a statistically significant relationship between the use of NGT and any of the postoperative complications, mortality, readmission, and hospital or resuscitation unit stay (Table 4).
Estimation of the risk for postoperative complications associated with the use of NGT.
| OR/Estimation | Inferior 95%CI | Superior 95%CI | Post hoc probability | |
|---|---|---|---|---|
| Stay in SRU (days) | 0.014 | 0.615 | 1.809 | 0.51 |
| Hospital stay (days) | −0.008 | −0.129 | 0.055 | 0.52 |
| UGI alteration | 1.037 | 0.763 | 1.95 | 0.54 |
| Nausea | 0.664 | 0.027 | 3.644 | 0.62 |
| Vomiting | 0.634 | 0.027 | 3.353 | 0.64 |
| Atrial fibrillation | 0.705 | 0.05 | 3.114 | 0.62 |
| Chylothorax | 0.688 | 0.043 | 4.065 | 0.62 |
| ARDS | 1.316 | 0.574 | 8.862 | 0.64 |
| Pleural effusion | 1.074 | 0.689 | 2.73 | 0.57 |
| Pneumonia | 0.974 | 0.359 | 2.148 | 0.51 |
| Stenosis | 0.77 | 0.062 | 3.459 | 0.57 |
| Necrosis of the plasty | 0.977 | 0.088 | 11.47 | 0.51 |
| Anastomotic dehiscence | 0.958 | 0.082 | 10.97 | 0.51 |
| Readmission | 1.066 | 0.548 | 3.233 | 0.54 |
| 90-day mortality | 0.984 | 0.078 | 11.15 | 0.51 |
SRU: Surgery Recovery Unit; UGI: upper gastrointestinal series; ARDS: acute respiratory distress syndrome.
Although the recommendations of expert groups advocate the use of NGT for decompression after esophagectomy (if there are no contraindications) and removal 48h later,1,2,5 this study shows that avoiding its use during the postoperative period of an Ivor-Lewis esophagectomy and reconstruction with gastroplasty is a safe measure that does not increase hospital stay or postoperative morbidity and mortality.
The main objective of multimodal rehabilitation protocols is to reduce postoperative morbidity and mortality,6,7 thereby shortening hospital stay8 and improving the pre,9 intra10 and postoperative11 patient status. In most enhanced recovery protocols for esophageal surgery, the use of a tube for decompression during the postoperative period is a constant measure, with different criteria to indicate its removal, such as the absence of abdominal distension, dilation of the plasty or high discharge.6 These protocols justify the use of NGT on the basis that fluid accumulation and distension of the plasty (due to the division of the vagus nerves and disappearance of the lower esophageal sphincter during surgery) may increase the risk of anastomotic dehiscence and pneumonia due to bronchial aspiration, attributing a decrease in vomiting, pain and bronchial aspirations to the decompression of the plasty with the tube.12 However, the current evidence supporting its systematic use is scarce and sometimes contradictory. Thus, despite the fact that some controlled studies demonstrated a greater number of complications in patients without a decompression tube after esophagectomy,13,14 other studies support that early removal of the tube during the first postoperative day does not increase the risk of complications like pneumonia, anastomotic dehiscence, recurrent paralysis or gastrointestinal bleeding.15 A Chinese study, similar to the one developed at our hospital, included 90 patients (45 in each group) and showed that the degree of pharyngeal pain and the time until oral ingestion, time until the expulsion of air and hospitalization days were lower in the group that did not have a catheter, without a greater number of complications or postoperative vomiting.16 In a recent prospective and multicenter clinical trial17 with patients undergoing Ivor-Lewis esophagectomy in whom NGT was not placed during the postoperative period, it has been shown that the onset of early oral tolerance is not related to a greater number of complications compared to later initiation of tolerance.
A recent meta-analysis including seven well-designed studies with 608 patients treated with esophagectomy has concluded that immediate or early removal of the NGT does not increase the number of anastomotic dehiscences, pulmonary complications or postoperative mortality, as in our study, but it also reduces hospital stay.18
Although the use of a catheter is not usually associated with serious complications, certain morbidity could be caused by its use and placement, such as odynophagia or otalgia, lesions in the nasal mucosa, sinusitis, gastritis and epistaxis. Some groups report a higher rate of respiratory complications associated with the use of NGT,18 as the tube prevents bronchial secretions from being correctly emptied, favoring their accumulation and superinfection. Increased patient discomfort, postoperative ileus and hospitalization time have also been reported.19,20 In this study, no differences were found in these variables regarding the use of NGT.
One of the supposed advantages of NGT is the reduction of nausea, vomiting and abdominal distension. However, similar to the results of this study, other recent studies have not found significant differences20–22 on this point.
The prevention of a possible anastomotic dehiscence is one of the basic pillars that has traditionally supported the use of NGT in the postoperative period of esophagectomy. However, in recent years this theory has been questioned, as the use of NGT has been attributed a higher incidence of anastomotic leakage according to some series.20 In this study, no significant differences were found in the appearance of anastomotic leak between both groups, and the percentage of dehiscence (2.3%) concurs with reports in the literature.23–25
The need to reposition an NGT during the postoperative period of an esophagectomy is rare, and when required it can be safely carried out without a high number of complications.5,12,18 In the experience presented, a single case required the placement of NGT due to persistent vomiting and paralytic ileus, which was done without associated complications.
As with morbidity, there have been no differences in postoperative mortality rates with or without routine NGT use,20 and the overall mortality presented in this study is consistent with acceptable figures after Ivor-Lewis esophagectomy.26,27
Some series have shown a reduction in costs and hospital stay with the omission of the use of NGT in patients treated with esophagectomy.28,29 In this study, however, no significant differences were found in the overall resuscitation unit or hospital stays between the groups.
Obviously, this study has some limitations that should be considered, such as the non-randomized retrospective design, the limited number of cases and the low frequency of certain adverse events studied. Also, there may be other confounding variables not studied that could influence the morbidity and mortality results and hospital stay of this complex surgery.
Therefore, based on the results obtained, it appears that the systematic use of NGT after esophagectomy can be routinely avoided without increasing the number of complications or their severity. Given the lack of clear evidence in this regard, prospective and randomized clinical trials with a larger sample size are necessary to provide greater scientific evidence.
Conflict of interestsThe authors have no conflict of interests.
Please cite this article as: Menéndez-Jiménez M, Bruna-Esteban M, Mingol F, Vaqué J, Hervás D, Álvarez-Sarrado E, et al. Uso de sonda nasogástrica en pacientes sometidos a esofaguectomía: ¿Un gesto innecesario? Cir Esp. 2020;98:598–604.