Robot-assisted thoracic surgery (RATS) is a rapidly expanding technique. In our study, we aimed to analyze the results of the process to adopt robotic surgery in our Department of Thoracic Surgery.
MethodsThis is an intention-to-treat analysis of a series of consecutive patients operated on using the RATS approach in our hospital from January 2021 to March 2022. Data were registered for patient characteristics, type of surgery, operative times, conversion rate, chest tube duration, length of hospital stay and complications.
The IBM SPSS® statistical software was used for the statistical analysis. A cumulative sum analysis of the operating time was performed to define the learning curve.
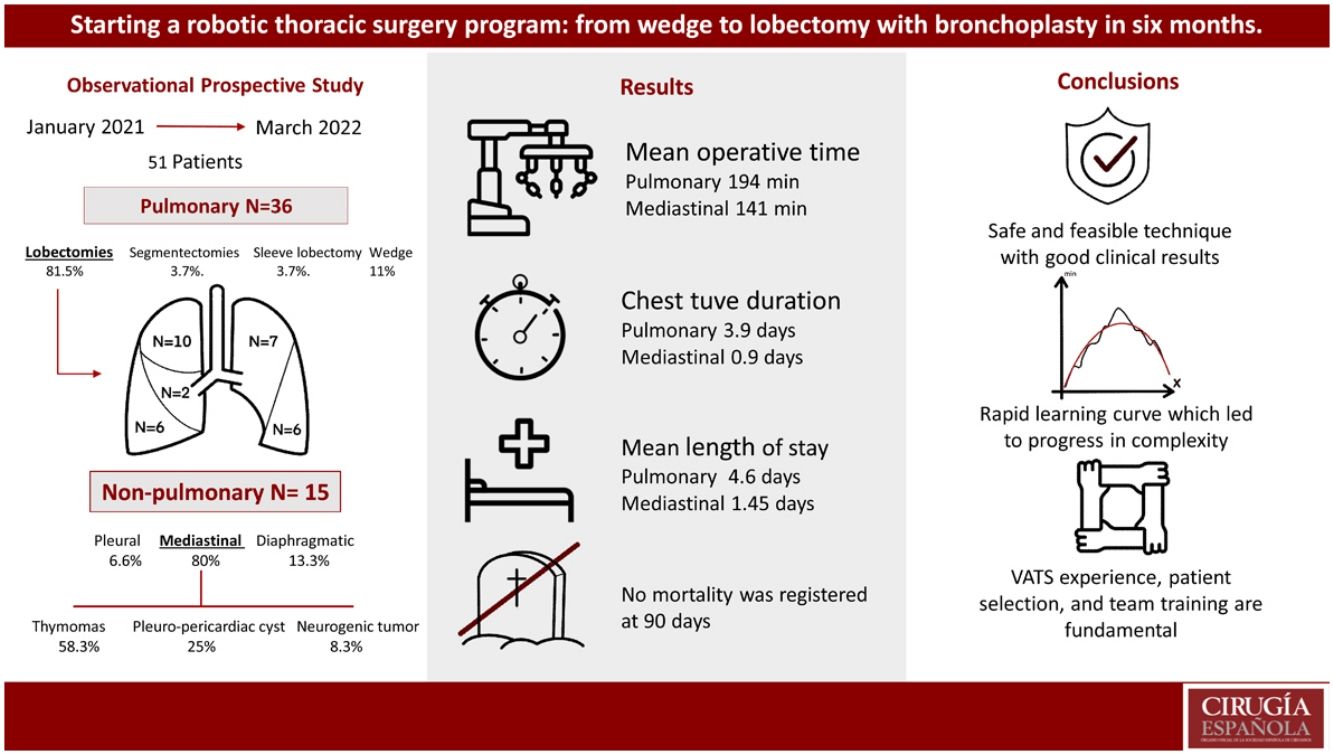
ResultsDuring the study period, 51 patients underwent robotic surgery, including pulmonary and non-pulmonary interventions. In addition, 15 patients (29.4%) underwent non-pulmonary interventions: one pleural (2%), 2 diaphragmatic (3.9%), and 12 mediastinal (23.5%). Among the mediastinal surgeries, one conversion was necessary (8.3%) for a complex vascular malformation, and 11 were completed by RATS, including 7 (58.3%) thymomas, 3 (25%) pleuro-pericardial cysts, and one (8.3%) neurogenic tumor. Mean operative time was 141 min (104–178), mean chest tube duration was 0.9 days (0–2), and mean length of stay was 1.45 days (1–2).
Thirty-six patients underwent lung surgery (70.6%). The complete RATS resections (34; 94.4%) included: 3 wedge resections (11.1%), 2 segmentectomies (3.7%), 28 lobectomies (81.5%), and one sleeve lobectomy (3.7%). Mean surgery time was 194.56 min (141–247), chest tube duration was 3.92 days (1–8), and length of stay was 4.6 days (1–8). Complications occurred in 4 patients (11.1%). No 90-day mortalities were registered.
ConclusionsThe implementation of RATS was achieved with good clinical results and operative times for all indications. A rapid learning curve was accomplished in short time. Previous VATS experience, patient selection, team training and program continuity are fundamental to successfully develop a RATS program.
La cirugía torácica asistida por robot (RATS) es una técnica en rápida expansión. Nuestro objetivo fue analizar el resultado del proceso de adopción de la cirugía robótica en nuestro Departamento de Cirugía Torácica.
MétodosEste es un análisis por intención de tratamiento de una serie de pacientes consecutivos operados mediante el método RATS en nuestro centro desde enero de 2021 a marzo de 2022. Se registraron las características de los pacientes, tipo de cirugía, tiempos operatorios, tasa de conversión, duración del drenaje torácico, estancia hospitalaria y complicaciones. Para el análisis estadístico se utilizó el software IBM SPSS®. Se realizó un análisis de suma acumulada del tiempo de operación para definir la curva de aprendizaje.
ResultadosDurante el periodo de estudio, 51 pacientes fueron sometidos a cirugía robótica.15 pacientes (29,4%) fueron sometidos a intervenciones no pulmonares: 1 pleural (2%), 2 diafragmáticas (3,9%) y 12 mediastínicas (23,5%). Entre las cirugías mediastínicas, fue necesaria una conversión (8,3%) por malformación vascular compleja y 11 fueron completadas por RATS, incluidos 7 (58,3%) timomas, 3 (25%) quistes pleuro-pericárdicos y 1 (8,3%) tumores neurogénicos. El tiempo operatorio medio fue de 141 minutos [104–178], la duración media del tubo torácico fue de 0,9 días [0–2] y la estancia media fue de 1,45 días [1–2].
36 pacientes tuvieron cirugías pulmonares (70,6%). Las resecciones RATS completas (34; 94,4%) incluyeron: 3 resecciones en cuña (11,1%), 2 segmentectomías (3,7%), 28 lobectomías (81,5%) y 1 lobectomía con broncoplastia (3,7%). El tiempo medio de cirugía fue de 194,56 minutos [141–247], la duración del tubo torácico fue de 3,92 días [1–8] y la estancia hospitalaria fue de 4,6 días [1–8]. No se registró mortalidad a los 90 días.
ConclusionesLa implementación de RATS se logró con buenos resultados clínicos y tiempos operatorios en todas las indicaciones. Se completó una rápida curva de aprendizaje en poco tiempo.
La experiencia previa en VATS, la selección de pacientes, la capacitación del equipo y la continuidad del programa son fundamentales para desarrollar con éxito un programa RATS.
While video-assisted thoracoscopy (VATS) has several advantages compared with open surgery, as smaller incisions, less pain, shorter hospital stay, quicker recoveries and a faster return to routine daily activity, it has some limitations, including lack of articulation of the instrument, two-dimensional visualization, and counterintuitive movement of the instrument. Robot-assisted thoracic surgery (RATS) is an evolving technique which has helped overcome some of these limitations. High-definition three-dimensional stereo video, improved ergonomics and tremor suppression are some of RATS benefits.1–3 In addition, the articulating robotic instrument is also better at performing procedures within deep and narrow spaces. These attributes may facilitate the resection of different elements, like hilar and mediastinal lymph nodes in robotic surgery compared with VATS.4–7
Such are some of the reasons why robotic surgery is growing exponentially worldwide. Since Melfi et al. firstly applied it to thoracic surgery in 2002,8 several authors have demonstrated the feasibility and safety of RATS for complex thoracic procedures.9–13 In recent years, multiple studies have compared other minimally invasive approaches, such as VATS, with RATS. In some of them, RATS was associated with less blood loss, lower conversion rates, a higher number of harvested lymph nodes, shorter postoperative chest tube drainage and less prolonged hospital stays.14–19
Despite reported benefits a slow implementation rate of RATS programs is being observed across Europe. In Spain, according to the work published by Varela et al., robot-assisted thoracic surgery is not implemented in most Thoracic Surgery Departments. This occurs in some reason because there is a reticence to go through a learning curve while the clinical and economic benefits are still under evaluation.20 Not only that, the increasing demand for RATS, requires structured and standardized training modules that will translate into efficient programs with good short-term results. Accordingly, meticulous and continuous analysis of the programs is necessary to demonstrate their effectiveness; some use learning curves to evaluate that.21,22
In the present study we want to analyze and evaluate initial clinical results, surgical times and learning curves after initiating a Robotic-Assisted Thoracic Surgery Program at our center.
MethodsThis is an intention to treat analysis of a series of consecutive patients operated on using Robotic assisted thoracic surgery approach in our center from January 2021 to March 2022. Da Vinci™ Xi surgical system (Intuitive, California, USA/ Abex Excellence Robotics, Spain) was used in all cases.
Demographics and patients’ characteristics, including age, gender, comorbidities, and functional status in case of lung resection, were duly registered before surgery. Patients were classified into four groups according to the type of surgery. Mediastinal surgery, including anterior tumors (thymoma and pleuro-pericardiac cyst) and posterior tumors (neurogenic and bronchogenic cyst); pleural surgery, including parietal and visceral pleura; lung surgery: including all lung resections, anatomic and non-anatomic; Fourth group was reserved for other interventions as diaphragmatic.
In general terms, the selection criteria used do not differ from those used for VATS in our hospital. In the case of pulmonary interventions, tumors up to clinical stage IIB were selected according to the 8th edition of the TNM. In the case of mediastinal lesions, full capsulated nodules with no image of invasion of mediastinal structures in computed tomography (CT) scan and/or magnetic resonance imaging (MRI) were selected for RATS approach. Likewise, in the other two groups, patients with lesions without involvement of central structures that could be resected by a minimally invasive approach were included. However, in order to achieve a safe program, start first cases selected for robotic surgery were small posterior mediastinal tumors, thymomas and non-anatomic lung resections which required lymphadenectomy. After that, progression to major lung resections was done starting with lower lobes previously diagnosed for non-small cell lung cancers.
Intraoperatively, surgical time, blood loss, rate of conversion, reason for conversion, final used approach (VATS or open surgery), and air leak were registered. Postoperatively, need and length of stay at intensive care unit (ICU) was registered. Complications and outcome at discharge were also registered. Day of chest tube removal and day of discharge were registered to calculate days of chest tube and total length of stay (LOS).
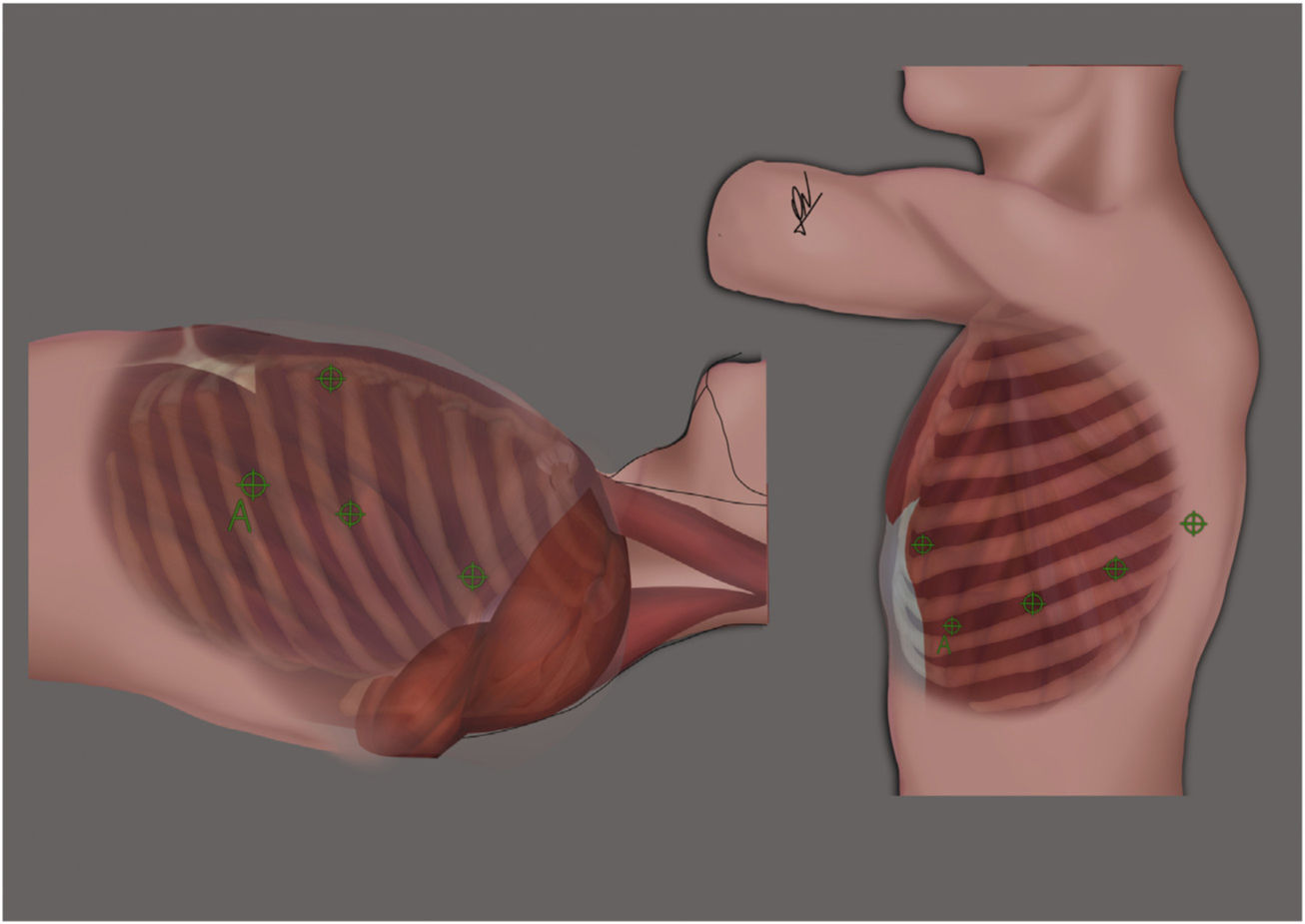
Lung surgeryLung surgeries were performed in the lateral decubitus position, with lateral flexion of the thorax. All robotic ports were placed according to Fig. 1(right) and CO2 insufflation at low pressure and flow was started (Pressure 5, Flow 5).
Left: Port placement for RATS thymectomy. 4 ports were used. One port was inserted in the third intercostal space and two in the fifth intercostal space. The assistance was placed at the level of the sixth intercostal space. Right: Port placement for RATS lung resection. All ports including assistance (A) were placed in the eighth intercostal space, except for the anterior port, which was placed in sixth intercostal space.
Specific analysis of lung interventions was done. Diagnostic for lung surgery was registered before intervention whether it was for lung metastasis, solitary lung nodule or diagnosed primary lung cancer. Thoracoscopic tunnel technique for anatomical resections was the preferred technique for dissection. Major anatomic resections were always completed by systematic nodal dissection. Type and extension of lung surgery were recorded. Extended resections characteristics were registered when necessary. A chest tube was placed at the end of surgery and total blood loss and air leak after extubation were registered.
Mediastinal surgeryFor the mediastinal approach, the patients were placed in supine decubitus position with partial elevation of the left hemithorax at 45°. Three robotic ports were placed according to Fig. 1(left) and CO2 insufflation at low pressure and flow was started (Pressure 5, Flow 5). For radical thymectomies resections started with a full dissection anterior to the left phrenic nerve and posterior to the mammary artery until identification of innominate vein. Thymic veins were divided either using clips or bipolar electrocoagulation. Dissection was completed all through the right phrenic nerve.
ConversionWe consider conversion when the surgery started by RATS but ended using another approach (VATS or open surgery) for any reason: emergent, technical or oncologic. The final surgery and outcome were registered and included in our database regardless.
Surgical timeSurgical time is the time span from skin opening to skin closure. It includes port placement, robot docking and targeting, console time, specimen retrieval and skin closure.
Statistical analysisResults are presented as n (proportion), mean ± standard deviation or median [interquartile range] as appropriate. The IBM SPSS® statistical software was used for statistical analysis. For the analysis of the learning curves measuring the surgical time, in lobectomies and thymectomies we resorted to the CUSUM (Cumulative Sum) graphs, which are widely used for quality control and best adapt to the monitoring of clinical-care processes.21,22
ResultsDuring the study period, 51 patients underwent thoracic robotic surgery in our department. Demographics and characteristics are summarized in Table 1. The surgeries performed were: 15 (29.4%) non-pulmonary interventions including 1 pleural (2%), 2 diaphragmatic (3.9%) and 12 mediastinal (23.5%). 36 patients had pulmonary surgery (70.6%).
All RATS patients (n = 51) demographics and characteristics.
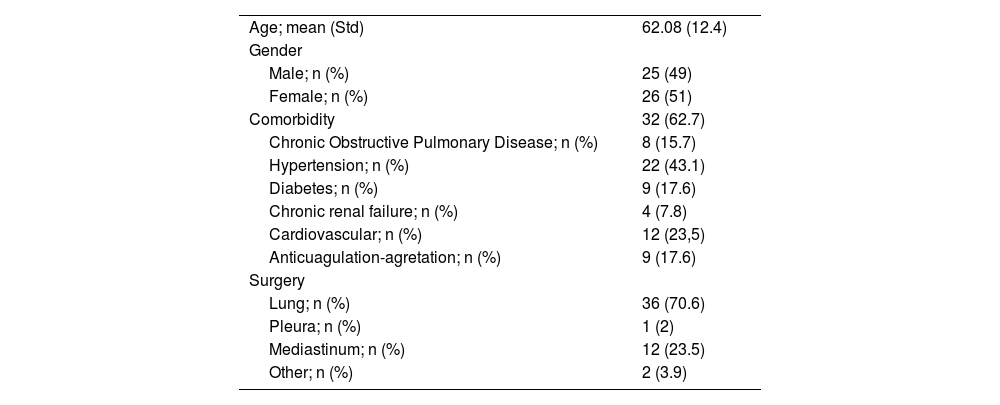
| Age; mean (Std) | 62.08 (12.4) |
| Gender | |
| Male; n (%) | 25 (49) |
| Female; n (%) | 26 (51) |
| Comorbidity | 32 (62.7) |
| Chronic Obstructive Pulmonary Disease; n (%) | 8 (15.7) |
| Hypertension; n (%) | 22 (43.1) |
| Diabetes; n (%) | 9 (17.6) |
| Chronic renal failure; n (%) | 4 (7.8) |
| Cardiovascular; n (%) | 12 (23,5) |
| Anticuagulation-agretation; n (%) | 9 (17.6) |
| Surgery | |
| Lung; n (%) | 36 (70.6) |
| Pleura; n (%) | 1 (2) |
| Mediastinum; n (%) | 12 (23.5) |
| Other; n (%) | 2 (3.9) |
36 patients underwent lung surgery with a median age of 65.56 (SD 11.2) and a gender distribution of 19 (52.7%) females and 17 (47.2%) males. Functional status are summarized in Table 2.
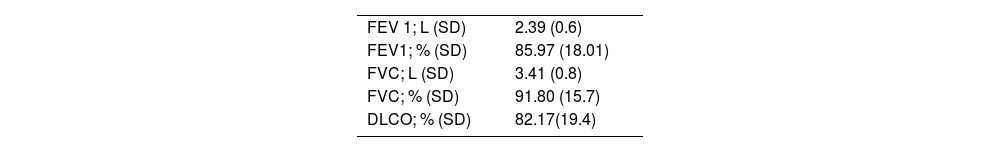
Preoperative pulmonary function tests for RATS lung resections (n = 36).
| FEV 1; L (SD) | 2.39 (0.6) |
| FEV1; % (SD) | 85.97 (18.01) |
| FVC; L (SD) | 3.41 (0.8) |
| FVC; % (SD) | 91.80 (15.7) |
| DLCO; % (SD) | 82.17(19.4) |
FEV1: Forced expiratory volume; FVC: Forced vital capacity; DLCO: Diffusion capacity of lung for carbon monoxide.
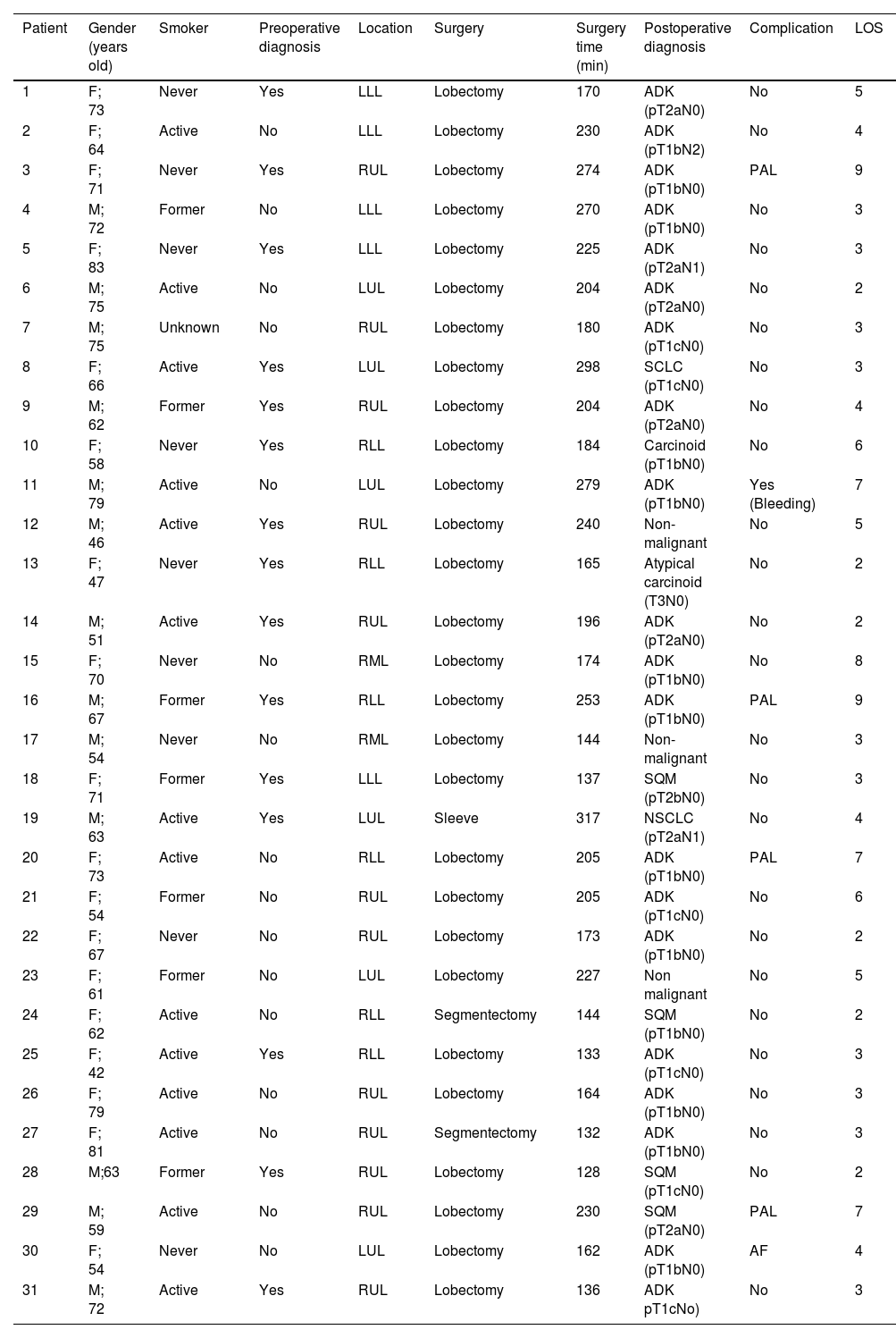
Completed RATS resections (34; 94.4%) included: 3 wedge resections (11.1%), 2 segmentectomies (3.7%), 28 lobectomies (81.5%), and 1 sleeve lobectomy (3.7%). Conversion to thoracotomy was necessary in two cases (5.6%) for oncological reasons; 1 patient had chest wall invasions, and the other presented hiliar lymph node with main pulmonary artery infiltration requiring a pneumonectomy.
After excluding wedge resections and conversion surgeries, 31 anatomic lung resections were performed by RATS during the study period. Type of surgery and distribution are summarized in Table 3. Mean surgical time for those patients who completed RATS lobectomy was 212.42 min [range 137–317], mean chest tube duration was 3.79 days [range 1–9] and mean length of stay was 4.46 days [range 2–9]. 12 patients (38.7%) spent the immediate postoperative period in the ICU. Their average stay at ICU was 1 day. When upper and non-upper lobectomies surgical times were compared no differences were detected (208 ± 56 vs 187 ± 45 min respectively; vs P = .2). 48% of patients (15) had a preoperative diagnostic and 16 patients (52%) needed intraoperative diagnostic; however, it did not affect surgical time (204 ± 61 min vs 195 ± 43 min, P = .6). Complications occurred in 4 patients (11.1%), 3 cases of air leak were registered and treated conservatively, and one patient required VATS re-interventions for bleeding. No mortality was registered at 90 days. Readmission was necessary in 3 cases (12.5%), 1 because of gastric bleeding, 1 for pain management and 1 because of wound infection.
Patients' characteristics.
| Patient | Gender (years old) | Smoker | Preoperative diagnosis | Location | Surgery | Surgery time (min) | Postoperative diagnosis | Complication | LOS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F; 73 | Never | Yes | LLL | Lobectomy | 170 | ADK (pT2aN0) | No | 5 |
| 2 | F; 64 | Active | No | LLL | Lobectomy | 230 | ADK (pT1bN2) | No | 4 |
| 3 | F; 71 | Never | Yes | RUL | Lobectomy | 274 | ADK (pT1bN0) | PAL | 9 |
| 4 | M; 72 | Former | No | LLL | Lobectomy | 270 | ADK (pT1bN0) | No | 3 |
| 5 | F; 83 | Never | Yes | LLL | Lobectomy | 225 | ADK (pT2aN1) | No | 3 |
| 6 | M; 75 | Active | No | LUL | Lobectomy | 204 | ADK (pT2aN0) | No | 2 |
| 7 | M; 75 | Unknown | No | RUL | Lobectomy | 180 | ADK (pT1cN0) | No | 3 |
| 8 | F; 66 | Active | Yes | LUL | Lobectomy | 298 | SCLC (pT1cN0) | No | 3 |
| 9 | M; 62 | Former | Yes | RUL | Lobectomy | 204 | ADK (pT2aN0) | No | 4 |
| 10 | F; 58 | Never | Yes | RLL | Lobectomy | 184 | Carcinoid (pT1bN0) | No | 6 |
| 11 | M; 79 | Active | No | LUL | Lobectomy | 279 | ADK (pT1bN0) | Yes (Bleeding) | 7 |
| 12 | M; 46 | Active | Yes | RUL | Lobectomy | 240 | Non-malignant | No | 5 |
| 13 | F; 47 | Never | Yes | RLL | Lobectomy | 165 | Atypical carcinoid (T3N0) | No | 2 |
| 14 | M; 51 | Active | Yes | RUL | Lobectomy | 196 | ADK (pT2aN0) | No | 2 |
| 15 | F; 70 | Never | No | RML | Lobectomy | 174 | ADK (pT1bN0) | No | 8 |
| 16 | M; 67 | Former | Yes | RLL | Lobectomy | 253 | ADK (pT1bN0) | PAL | 9 |
| 17 | M; 54 | Never | No | RML | Lobectomy | 144 | Non-malignant | No | 3 |
| 18 | F; 71 | Former | Yes | LLL | Lobectomy | 137 | SQM (pT2bN0) | No | 3 |
| 19 | M; 63 | Active | Yes | LUL | Sleeve | 317 | NSCLC (pT2aN1) | No | 4 |
| 20 | F; 73 | Active | No | RLL | Lobectomy | 205 | ADK (pT1bN0) | PAL | 7 |
| 21 | F; 54 | Former | No | RUL | Lobectomy | 205 | ADK (pT1cN0) | No | 6 |
| 22 | F; 67 | Never | No | RUL | Lobectomy | 173 | ADK (pT1bN0) | No | 2 |
| 23 | F; 61 | Former | No | LUL | Lobectomy | 227 | Non malignant | No | 5 |
| 24 | F; 62 | Active | No | RLL | Segmentectomy | 144 | SQM (pT1bN0) | No | 2 |
| 25 | F; 42 | Active | Yes | RLL | Lobectomy | 133 | ADK (pT1cN0) | No | 3 |
| 26 | F; 79 | Active | No | RUL | Lobectomy | 164 | ADK (pT1bN0) | No | 3 |
| 27 | F; 81 | Active | No | RUL | Segmentectomy | 132 | ADK (pT1bN0) | No | 3 |
| 28 | M;63 | Former | Yes | RUL | Lobectomy | 128 | SQM (pT1cN0) | No | 2 |
| 29 | M; 59 | Active | No | RUL | Lobectomy | 230 | SQM (pT2aN0) | PAL | 7 |
| 30 | F; 54 | Never | No | LUL | Lobectomy | 162 | ADK (pT1bN0) | AF | 4 |
| 31 | M; 72 | Active | Yes | RUL | Lobectomy | 136 | ADK pT1cNo) | No | 3 |
F: female; M: male; RUL: rigth upper lobectomy, RML: rigth middle lobectomy; RLL: rigth lower lobectomy; LUL: left upper lobectomy; LLL: left lower lobectomy; ADK: adenocarcinoma; SCLC: small cell lung cancer; SQM: squamous cell carcinoma; PAL: Persistent air leak; AF: atrial fibrillation; LOS: length of stay.
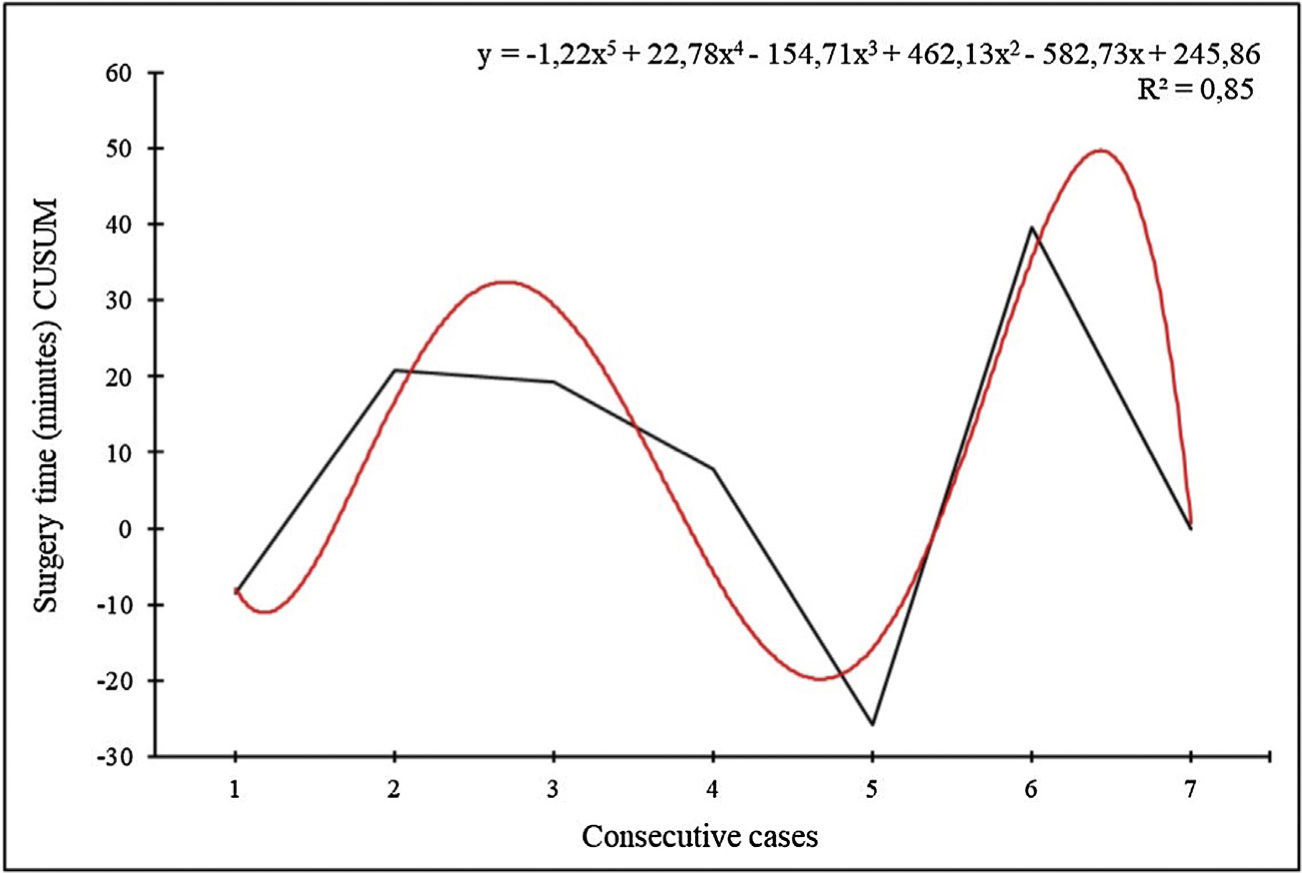
The data of the CUSUM curve for the robotic surgery time of the lobectomies were adjusted to a polynomial of order 2 (Fig. 2). In our series, the learning curve was completed with 23 procedures.
Mediastinal surgery12 (23.5%) patients underwent mediastinal surgery with a median age of 52 (SD 11.4) and a gender distribution of 6 (50%) females and 6 (50%) males. 11 surgeries were completed by RATS. Conversion was necessary in one case (8.3%) because pericardial, left lung and innominate vein involvent of a complex vascular malformation of the mediastinum.
Among RATS completed surgeries the diagnoses were, 7 (58.3%) were thymomas, 3 (25%) pleuro-pericardiac cysts and 1 (8.3%) neurogenic tumor. Mean time was 141 min [104–178], mean chest tube duration was 0.9 days [0–2] and mean length of stay was 1.45 days.1,2 No complications were registered.
Learning curve for thymectomiesThe data of the CUSUM curve for the time of robotic surgery of the thymectomies were adjusted to a polynomial of order 5 (Fig. 3).
DiscussionWithin 15 months, we performed 51 surgeries, achieving an increasing level of complexity with no impact on surgical times and a low percentage of complications and conversions. Anatomical lung resections were the most common procedures with 31 surgeries, allowing us to consolidate our learning curve after 23 cases. We have also performed surgeries for mediastinal, pleural, and diaphragmatic pathologies with no complications and only one conversion.
The GEVATS group (Spanish Group of Video-Assisted Thoracic Surgery), analyzed the Spanish national database23 and reported a rate of morbidity, mortality and conversions comparable to our first cases of RATS. When evaluating our postoperative hospital stay in pulmonary resections, it averaged 4.6 days (SD 2–9) compared to 5 days (SD 4–7) in the GEVATS series published in 2021.24 In the other hand, our results are far from more experienced groups. Travis C. Geraci et al. published, a 53% rate of discharge at postoperative day one from a total of 253 robotic anatomic pulmonary resections.25
Regarding the defined learning curve for RATS lobectomies, we evidenced a learning curve completed at case 23 based on surgical time. Previous articles determined that the learning curve for anatomical lung resections is completed after 14–34 cases when analyzing the surgical time.21,26–28 However, the learning curve can also be measured using other parameters. Meyer et al. used mortality and surgeon comfort, and set the learning curve at 20, and 19 cases, respectively.27
Most of the procedures we have performed are pulmonary resections (70.6%). From our experience with this type of procedure, we can extract three conclusions worth taking into account when starting a robotics program.
First, previous experience in minimal invasive surgery might play an important role. At the time of starting the program we had a rate of VATS near to 80% of all anatomical resections. In this direction Merritt et al. suggested that, concepts from previous VATS experience might be transferred to RATS and help to reduce surgical times in a faster and safer way.9,29 For example, our previous experience using the tunnel technique for anatomical lung resections, described by Decaluwe30 on VATS resections permitted us to use it on RATS.31 Tunnel technique requires less parenchymal manipulation and reduces accidental tears happening during the first RATS cases.
Second, selection of patients is crucial to improve results. We progressively increased complexity starting with small non pulmonar tumors, continuing to thymomas and non anatomical lung resections to end up carrying out anatomical lung resections. When starting anatomical lung resections we find recommendable to avoid large (>5 cm tumors) and central (in direct contact with central structures) tumors. In contraposition, selection of previously diagnosed peripheral tumors allowed us to complete a safe and steady learning curve minimizing intraoperative time and reducing loss of CO2 as a result of the retrieval of the specimen for diagnosis. We also preferred non-upper lobectomies for the first cases because they are less technically demanding and unexpected vascular injuries are easier to repair. Lower lobectomies required less surgical time in our series, although this data is not statistically significant.
Third, training is essential to achieve good results. Field and console surgeons trained in animals and cadaveric models before clinical application. Virtual simulation was also used adding advantage when implementing this new technique.17,32–34 As stated by Shahim et al., we used dual console and the guidance of a mentor for the clinical implementation of the program. It is also important to achieve consistency and regularity in the number of surgeries to maintain high standards within the surgical team.33,35,36 In fact, something we experienced is that, by performing weekly sessions of robotic surgery with a close-trained team, within six months we were able to advance from minor resections (Wedge) to left lower sleeve lobectomy.
Taking a glimpse to the horizon we are convinced that new tools such as three-dimensional computed tomography reconstruction for operative planning in anatomical segmentectomies,37 novel variations on the current approach (e.g., the use of a single work port38) and the development of new surgical robotic systems,39 announce an encouraging future for this technique and can contribute to improving results and reducing costs in the coming years.40
LimitationsBeyond what has been described in this paper we have to point out some limitations. This is a non-randomized single center series of consecutive cases and the results and conclusions found in our study may not be completely applicable in other institutions. Furthermore, selection bias makes comparison with GEVATS series not feasible. However, as we stated before, selection is one of the cornerstones for a successful RATS program take off and GEVATS results can be a goal.
ConclusionsIn our experience after starting RATS program, we achieved good clinical results and operative times in all mediastinal, pleural, pulmonary and diaphragmatic surgeries. In addition, we observed a fast learning curve that led to greater complexity in a short time for anatomical lung resections.
In our opinion, prior VATS experience, patient selection and team training have been essential to achieve these results and to successfully develop a RATS program in thoracic surgery.
Financial supportThis research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflict of interestNone.
Please cite this article as: Paglialunga P, Boada M, Guzmán R, Sanchez-Lorente D, Guirao A, Bello I, et al. Inicio de un programa de cirugía torácica robótica: de resección en cuña a lobectomía con broncoplastia en seis meses. Conclusiones iniciales. Cir Esp. 2023.