A female patient, 42 years of age, a smoker, and with no other history of interest, presented with chest pain coinciding with her menstrual periods. The patient had been under the care of the digestive department since 2007 for epigastric discomfort and had been diagnosed with a subcarinal tumour suspected to be an oesophageal duplication cyst. Among the tests performed were:
- -
Chest CT: a hypodense lesion which was round and well-defined from the tracheal carina. Possible oesophageal duplication.
- -
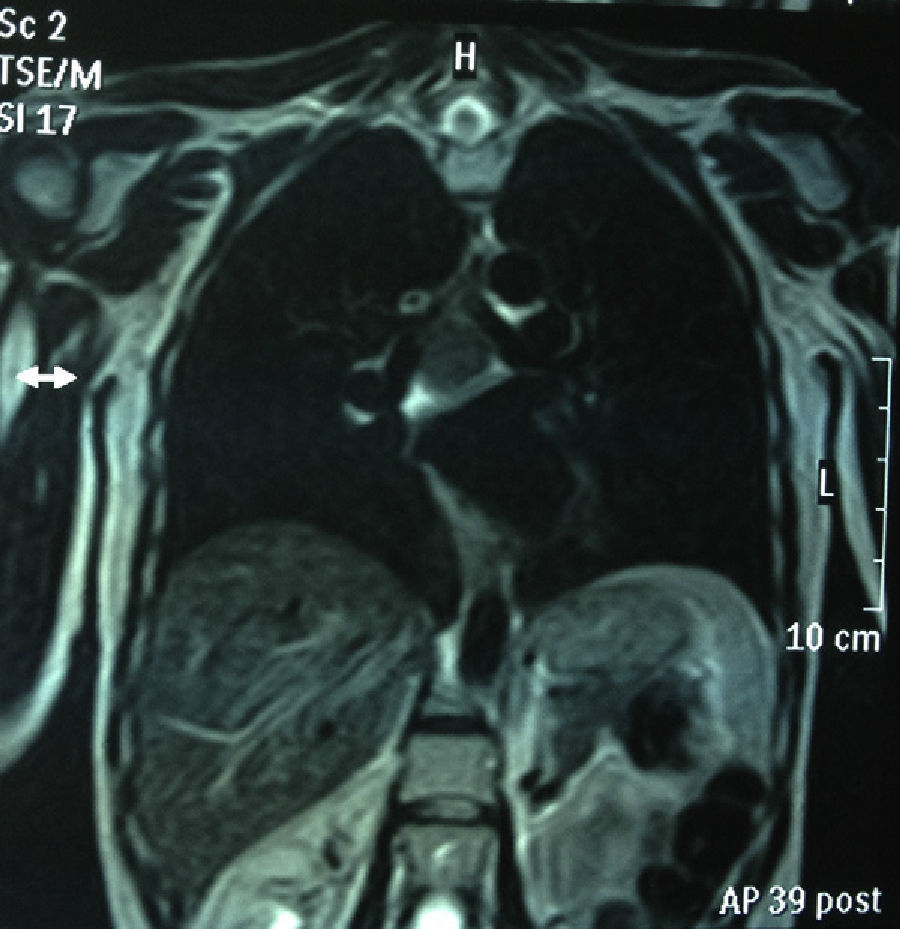
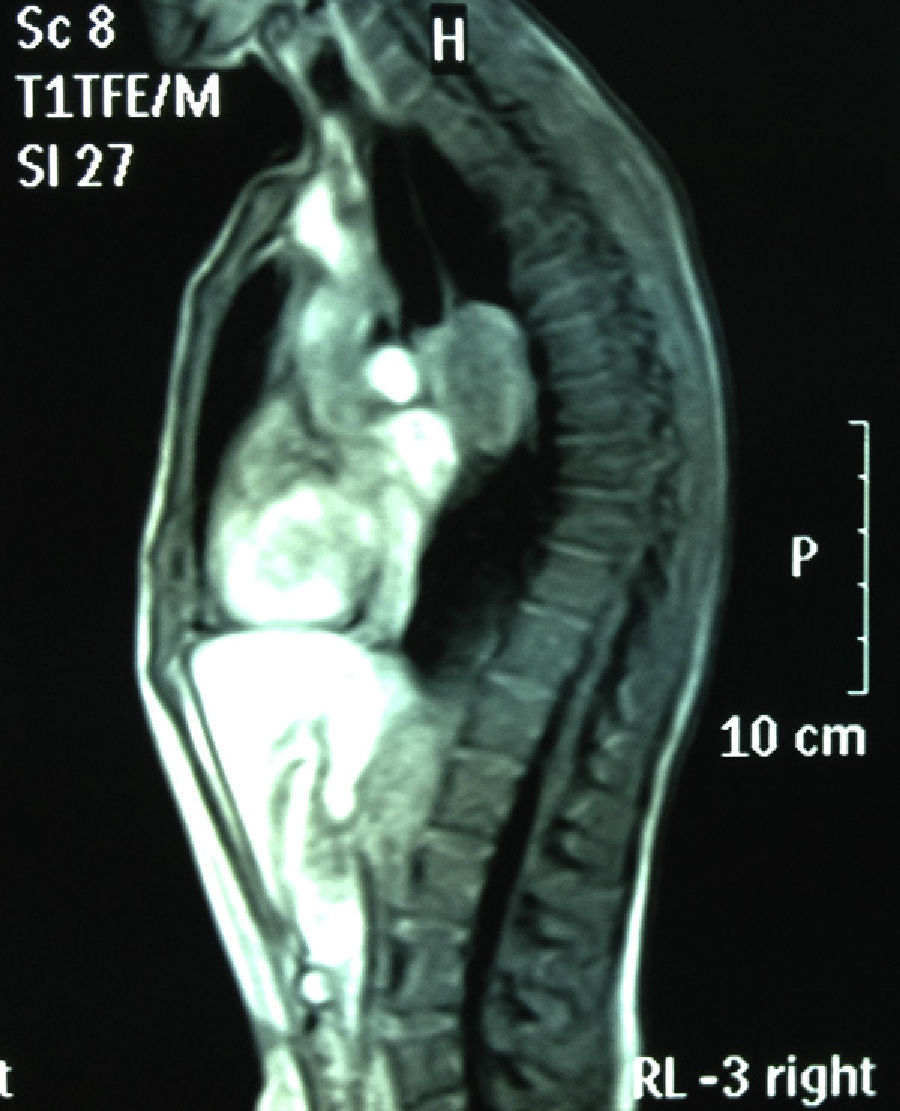
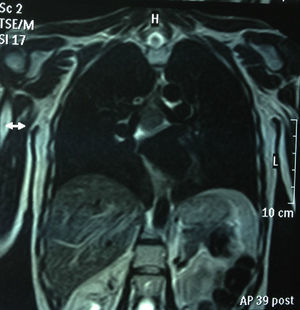
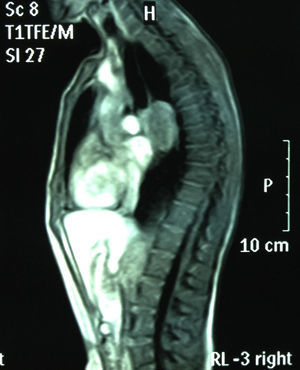
Chest MRI: subcarinal mediastinal mass of 45mm, hyperintense on T1 (Figs. 1 and 2).
- -
Echoendoscopy: oesophageal mucosa-dependent cystic lesion.
- -
Spirometry: FVC: 4.010ml (108%), FEV1: 3280ml/s (113%).
With the diagnosis of a suspected oesophageal duplication cyst, surgical treatment by resection was recommended.
A right-sided thoracotomy was performed under general anaesthetic and orotracheal intubation. A subcarinal mediastinal tumour of a hard consistency was found in the middle third of the oesophagus. It was resected with part of the oesophageal muscle, reinforcing the oesophagus with interrupted sutures in the unresected muscle layer. There was no evidence of spread to the oesophageal mucosa.
The report was sent to pathology department and the study showed a mediastinal gastrointestinal stromal tumour (GIST) of 6cm×3.5cm×3.3cm, with diffuse muscle differentiation, well- defined, with no necrosis, mitosis, or atypia (mitosis<5). A Giemsa stain was performed and interstitial mastocytes were observed (+). Immunophenotypic analysis of the tumour was: smooth muscle actin (+++), S-100: negative, Ki-67: (low proliferation rate <5%), CD-34 (+) in vessels, c-kit: +. Moderate risk (Fletcher 2002).
In 1983, the concept of a gastrointestinal stromal tumour was introduced by Mazur and Clack,1 until then GISTs were classified as leiomyoma, leiomyoblastoma or leiomyosarcoma. Thanks to advances in molecular biology and immunohistochemistry they have been defined as rare mesenchymal tumours, originating in the interstitial cells of Cajal. These cells express a mutant membrane receptor with anomalous tyrosine kinase (c-kit or CD117) which causes unregulated cellular proliferation. They tend to be asymptomatic, and are usually discovered incidentally during endoscopy or radiographic tests.2 GISTs are found predominantly in the stomach and the intestine, and rarely present in the oesosphagus. A GIST in the posterior mediastinum generally originates in the oesophagus, as occurred in this case. Although two cases of GISTs in the posterior mediastinum have been published,3,4 in this location differential diagnoses with neurogenic tumour, gastroesophageal duplication cyst and oesophageal leiomyoma should also be included. Mediastinal location is rare and the subcarinal presentation has not been reported to-date. Broad-based tumour growth in this location can cause compression symptoms such as dyspnoea, although our patient's symptoms started with epigastralgia and chest pain. The site where these tumours present is a prognostic factor. GISTs originating in the stomach are therefore more indolent, whereas GISTs arising in the small intestine, colon, and oesophagus are more aggressive. Most GISTs have a good prognosis after surgical resection, but in advanced studies (defined by size and mitosis) they are associated with low survival and a poor response to chemotherapy. In terms of medical treatment, imatinib tyrosine kinase inhibitor has been demonstrated to be effective in controlling GIST, improving survival even in non-metastatic GIST.2 However, the adverse effects of this drug, must be taken into consideration such as tumour necrosis5 and bleeding which, although not very frequent, are typical with imatinib, especially in cases of highly malignant GISTs, such as giant GIST of the duodenum (which are rare and have a major risk of haemorrhage), for which imatinib has to be given with the patient hospitalised.
GISTs have shown KIT genetic mutations, commonly in exon 11 and less frequently in exon 9. KIT mutations act as “guardians” in the development of GISTs. Immunophenotypic analysis is important in classifying these tumours, so there are studies confirming that the Ki-676 proliferation rate could be an excellent prognostic survival marker in patients with c-kit+, and could be helpful in the selection of candidates for treatment with imatinib.
Aggressive surgical resection with negative margins has always been the treatment of choice for GIST, due to radio resistance and insensitivity to chemotherapeutic agents. But the advances described using imatinib imply that new, less aggressive, surgical techniques can be considered7 which offer similar survival, abandoning resection in favour of enucleation and thus calling aggressive gastroesophageal surgery into question due to its elevated risk.
There are studies on laparoscopic8 resection of gastrointestinal stromal tumours, which support this for wedge resections. There is no location where video-surgery is strictly contraindicated. The main disadvantages described are post-operative stenoses, in particular in the oesphago-gastric junction or pylorus. It is suggested that imatinib be used beforehand in the case of voluminous or unfavourably located tumours, in order to reduce the mass, enabling conservative surgery of the organ.
Please cite this article as: Arango Tomás E, Algar Algar FJ, Salvatierra Velázquez Á. Tumor subcarinal del estroma intestinal. Cir Esp. 2013;91:677–678.