The aim of this study is to determine the usefulness of the risk model developed by van Ramshorst et al., and a modification of the same, to predict the abdominal wound dehiscence's risk in patients who underwent midline laparotomy incisions.
Materials and methodsObservational longitudinal retrospective study. Sample: Patients who underwent midline laparotomy incisions in the General and Digestive Surgery Department of the Sabadell's Hospital – Parc Taulí’s Health and University Corporation, Barcelona, between January 1, 2010 and June 30, 2010. Dependent variable: Abdominal wound dehiscence. Independent variables: Global risk score, preoperative risk score (postoperative variables were excluded), global and preoperative probabilities of developing abdominal wound dehiscence.
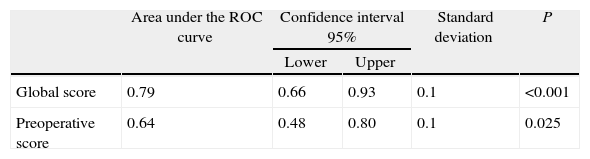
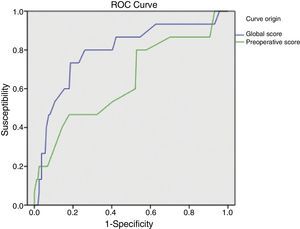
ResultsSample: 176 patients. Patients with abdominal wound dehiscence: 15 (8.5%). The global risk score of abdominal wound dehiscence group (mean: 4.97; CI 95%: 4.15–5.79) was better than the global risk score of No abdominal wound dehiscence group (mean: 3.41; CI 95%: 3.20–3.62). This difference is statistically significant (P<.001). The preoperative risk score of abdominal wound dehiscence group (mean: 3.27; CI 95%: 2.69–3.84) was better than the preoperative risk score of No abdominal wound dehiscence group (mean: 2.77; CI 95%: 2.64–2.89), also a statistically significant difference (P<.05). The global risk score (area under the ROC curve: 0.79) has better accuracy than the preoperative risk score (area under the ROC curve: 0.64).
ConclusionThe risk model developed by van Ramshorst et al. to predict the abdominal wound dehiscence's risk in the preoperative phase has a limited usefulness. Additional refinements in the preoperative risk score are needed to improve its accuracy.
Nuestro trabajo pretende valorar la utilidad del modelo de riesgo de evisceración desarrollado por van Ramshorst et al., y una modificación del mismo, para predecir el riesgo de evisceración entre pacientes operados por laparotomía media.
Material y métodosEstudio observacional, longitudinal y retrospectivo. Muestra: pacientes operados por laparotomía media en la Corporación Sanitaria y Universitaria Parc Taulí (Barcelona), entre el 1 de enero y el 30 de junio del 2010. Variable dependiente: evisceración. Variables independientes principales: los scores de riesgo global y preoperatorio (excluye variables postoperatorias), y las probabilidades de evisceración global y preoperatoria.
ResultadosMuestra: 176 pacientes. Eviscerados: 15 (8,5%). La media del score global de riesgo del grupo Evisceración: 4,97 (IC95%: 4,15-5,79) es mayor que la del grupo No evisceración: 3,41 (IC95%: 3,20-3,62), siendo esta diferencia estadísticamente significativa (p<0,001). La media del score preoperatorio de riesgo del grupo Evisceración: 3,27 (IC95%: 2,69-3,84) es mayor que la del grupo No evisceración: 2,77 (IC95%: 2,64-2,89), siendo esta diferencia estadísticamente significativa (p<0,05). El score global de riesgo (área bajo la curva ROC: 0,79) tiene mayor capacidad predictiva que el score preoperatorio de riesgo (área bajo la curva ROC: 0,64).
DiscusiónLa utilidad del modelo de riesgo desarrollado por van Ramshorst et al. para predecir el riesgo de evisceración, durante el preopeatorio, entre pacientes operados por laparotomía media es limitada. La utilización del score preoperatorio requiere ajustes para mejorar su rendimiento pronóstico.
Abdominal wound dehiscence is a serious postoperative complication with a high morbimortality; it also entails increased patient treatment costs, due to reoperations, postoperative complications and prolonged hospital stay.1–8
van Ramshorst et al.1 developed and validated a risk model to help predict wound dehiscence risk for patients undergoing abdominal surgery.
An effective risk model would be useful for deciding which patients require modification of laparotomy closure techniques (e.g. use of mesh) to reduce the risk of abdominal wound dehiscence.
The aim of this study is to determine the usefulness of the risk model developed by van Ramshorst et al.1 to predict abdominal wound dehiscence risk and define risk groups among patients scheduled for surgery using midline laparotomy. We will also evaluate the usefulness of modifying the previous model (modified van Ramshorst).
Material and MethodsWe developed an observational, longitudinal, analytical and retrospective study where the sample studied were patients with a midline laparotomy performed in the General and Digestive Surgery Department Sabadell's Parc Tauli Hospital– Health and University Corporation, Barcelona, between 1st January and 30th June 2010. Patients operated for ventral hernias, incisional hernias, or other types of laparotomy were excluded, as were those who underwent surgery in other hospital departments.
The study's dependent variable was presentation of abdominal wound dehiscence. Main independent variables were: (1) global risk score (van Ramshorst et al.1) which is the total score of each independent variable score; (2) preoperative risk score (modified van Ramshorst) which is the total score of each preoperative variable score (postoperative variables of cough and wound infection were excluded); (3) the probability of overall dehiscence (van Ramshorst et al.1) calculated with the following logistic equation1: P=ex/(1+ex)×100%, where x=−8.37+(1.085×calculated overall risk score); and (4) the probability of preoperative dehiscence (modified van Ramshorst), which is calculated using the same equation, replacing the calculated overall risk score by the calculated preoperative risk score. Secondary independent variables with their respective risk scores were: age (40–50: 0.4; 50–70: 0.9; over 70: 1.1), gender (male: 0.7), history of chronic pulmonary disease (0.7), ascites (described in the surgical report or in imaging tests as: 1.5), jaundice (bilirubinanaemia >2.9mg/dL within 48 preoperative hours: 0.5), anaemia (haemoglobin <7.5g/dL in women and <8g/dL in men, within 48 preoperative hours: 0.7), emergency surgery (0.6), type of surgery (hepato-biliary: 0.7; oesophagus: 1.5; gastroduodenal: 1.4; small intestine: 0.9; colon: 1.4; vascular: 1.3), cough (registered in clinical reports: 1.4), wound infection (1.9).
Data were collected from: (1) the hospital computerized clinical work station; (2) our adverse events database, which includes all adverse events suffered by our patients since 20052,3 collected prospectively and (3) archived clinical histories. Patient data confidentiality was always maintained. An MS-Access database was constructed into which independent variable values and automatic calculations of risk scores, and the probability of abdominal wound dehiscence and global and preoperative scores of each patient could be recorded.
The statistics programme IBM SPSS Statistics version 19 for Windows was used for data analysis. The sample was divided into 2 subgroups for analysis: abdominal wound dehiscence and no abdominal wound dehiscence. We used percentages to describe the categorical variables and means with 95% confidence intervals and standard deviation to describe the continuous variables. In the analytical study, the categorical variables were analyzed with the χ2 test and the quantitative variables with the Students t-test for independent samples. Statistical significance was considered if P<.05. The predictive value of the risk model in our population was evaluated using ROC curves.
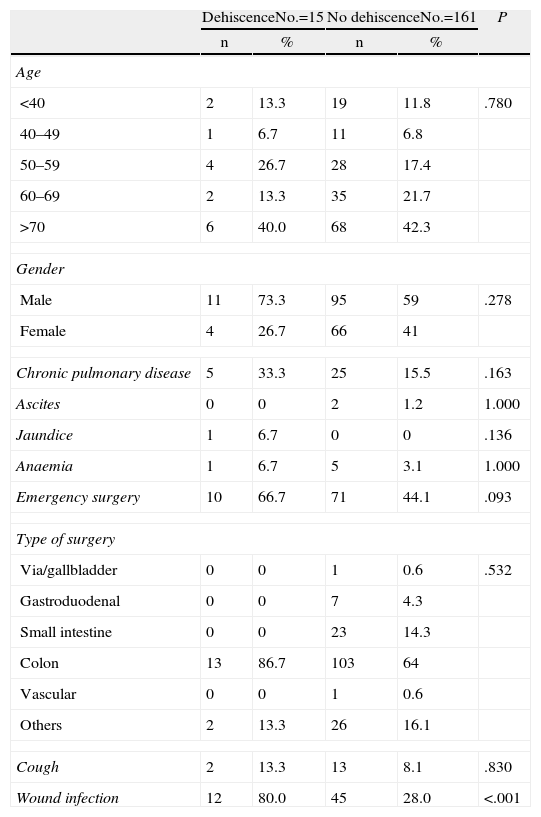
ResultsOur study included 176 patients who underwent midline laparotomy surgery, of which 15 presented abdominal wound dehiscence (8.5%). Descriptive analysis of the secondary independent variables is shown in Table 1.
Description of Preoperative Variables.
| DehiscenceNo.=15 | No dehiscenceNo.=161 | P | |||
| n | % | n | % | ||
| Age | |||||
| <40 | 2 | 13.3 | 19 | 11.8 | .780 |
| 40–49 | 1 | 6.7 | 11 | 6.8 | |
| 50–59 | 4 | 26.7 | 28 | 17.4 | |
| 60–69 | 2 | 13.3 | 35 | 21.7 | |
| >70 | 6 | 40.0 | 68 | 42.3 | |
| Gender | |||||
| Male | 11 | 73.3 | 95 | 59 | .278 |
| Female | 4 | 26.7 | 66 | 41 | |
| Chronic pulmonary disease | 5 | 33.3 | 25 | 15.5 | .163 |
| Ascites | 0 | 0 | 2 | 1.2 | 1.000 |
| Jaundice | 1 | 6.7 | 0 | 0 | .136 |
| Anaemia | 1 | 6.7 | 5 | 3.1 | 1.000 |
| Emergency surgery | 10 | 66.7 | 71 | 44.1 | .093 |
| Type of surgery | |||||
| Via/gallbladder | 0 | 0 | 1 | 0.6 | .532 |
| Gastroduodenal | 0 | 0 | 7 | 4.3 | |
| Small intestine | 0 | 0 | 23 | 14.3 | |
| Colon | 13 | 86.7 | 103 | 64 | |
| Vascular | 0 | 0 | 1 | 0.6 | |
| Others | 2 | 13.3 | 26 | 16.1 | |
| Cough | 2 | 13.3 | 13 | 8.1 | .830 |
| Wound infection | 12 | 80.0 | 45 | 28.0 | <.001 |
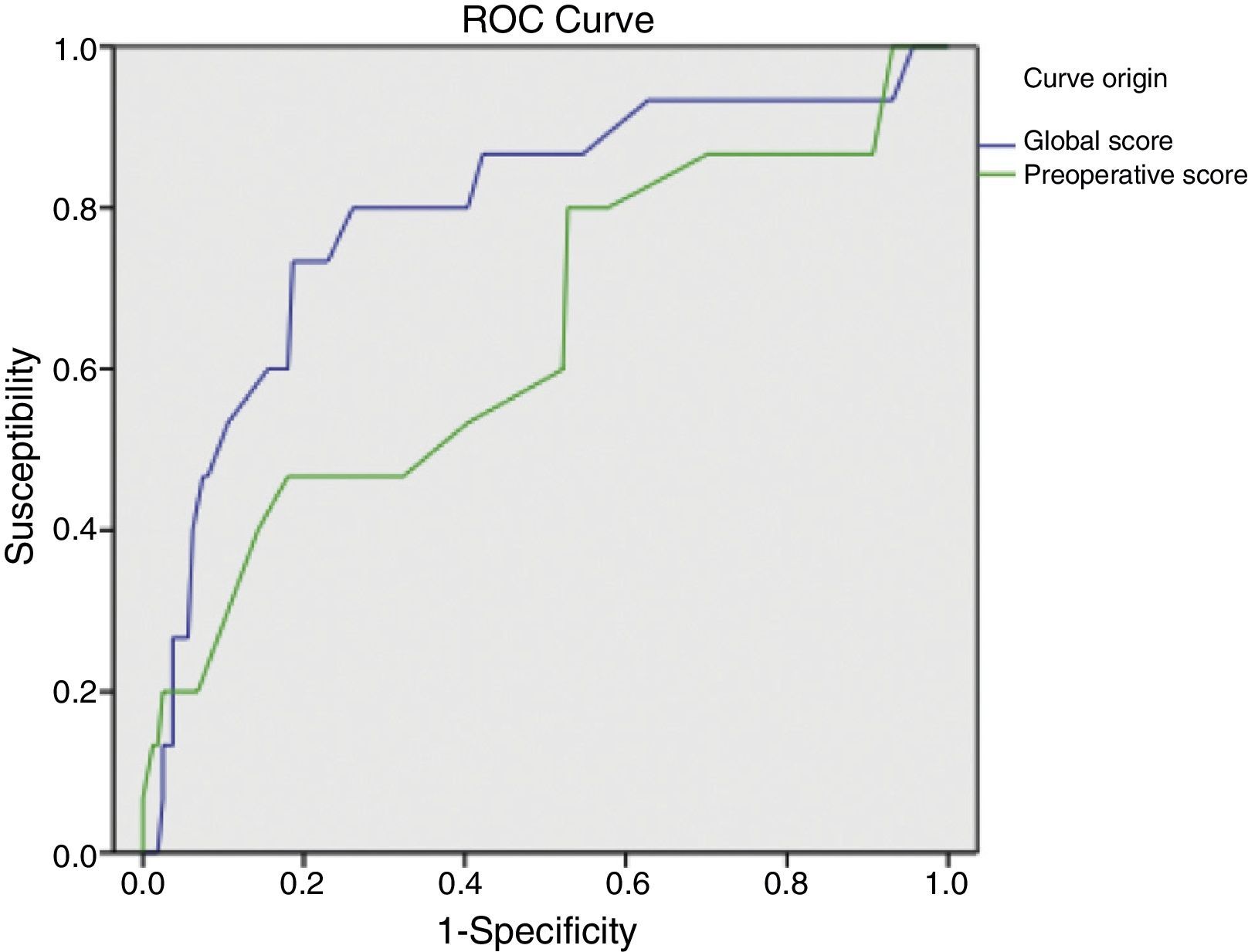
The global mean risk scored in the abdominal wound dehiscence group was 4.97 (CI 95%: 4.15–5.79) DE=1.5, whilst in the non-abdominal wound dehiscence group, the global mean risk score was 3.41 (CI 95%: 3.20–3.62) DE=1.4. The difference between the 2 groups is statistically significant (P<.001). Mean preoperative risk score in the abdominal wound dehiscence group was 3.27 (CI 95%: 2.69–3.84) DE=1.0; whilst in the non-abdominal wound dehiscence group mean preoperative risk score was 2.77 (CI 95%: 2.64–2.89) DE=0.8. The difference between the 2 groups is statistically significant (P<.05). Predictive capacity of both models may be appreciated in Fig. 1 and Table 2.
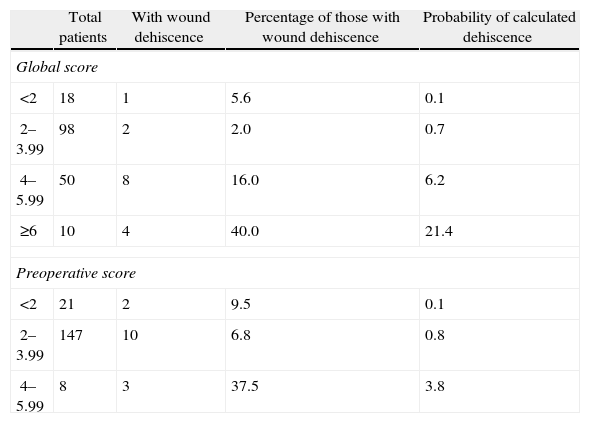
Table 3 shows the simple distribution in the different categories of global score or preoperative risk score proposed by van Ramshorst et al.1 The global score distribution by categories is statistically significant (P<.001), whilst the preoperative score is not (P>.05).
Classification of Global and Preoperative Scores of Abdominal Wound Dehiscence Risk.
| Total patients | With wound dehiscence | Percentage of those with wound dehiscence | Probability of calculated dehiscence | |
| Global score | ||||
| <2 | 18 | 1 | 5.6 | 0.1 |
| 2–3.99 | 98 | 2 | 2.0 | 0.7 |
| 4–5.99 | 50 | 8 | 16.0 | 6.2 |
| ≥6 | 10 | 4 | 40.0 | 21.4 |
| Preoperative score | ||||
| <2 | 21 | 2 | 9.5 | 0.1 |
| 2–3.99 | 147 | 10 | 6.8 | 0.8 |
| 4–5.99 | 8 | 3 | 37.5 | 3.8 |
In the last few years, 2 groups of authors have developed studies to determine abdominal wound dehiscence risk scores. The first, proposed by Webster et al.,9 included 12 variables, almost half of which were postoperative. This reduced the score capacity to predict a future abdominal wound dehiscence. The second, developed by van Ramshorst et al.,1 included 10 variables, 2 of which were postoperative. We have chosen the latter risk score to validate our work.
The first criticism of the score presented by van Ramshorst et al.1 is the use of the surgical wound infection variable since, as we have seen in our study and in the literature, this variable is a main risk factor for abdominal wound dehiscence,1,4,5,10,11 but has a very serious limitation as a predictive factor, because its presentation is in the postoperative period.1,9
Application of the overall abdominal wound dehiscence risk score (van Ramshorst et al.1) obtained statistically significant differences among the subgroups and the ROC curve expressed good predictive capacity, but when we wished to evaluate the probability of abdominal wound dehiscence after classifying the sample in overall risk groups, we noted that, although the calculated probability of abdominal wound dehiscence progressively increases to a higher global score value, the real percentage of abdominal wound dehiscence within each group is higher than the calculated probability. The equation therefore underestimates the abdominal wound dehiscence risk in our sample. This could be explained by the low incidence of abdominal wound dehiscence in the sample which van Ramshorst et al.1 used to validate the risk score (2.8%), and because our study sample (which included only midline laparotomies) is not comparable to that used by van Ramshorst et al.1 (which includes any type of laparotomy), or because there are differences in the efficacy of the surgical technique we used for abdominal wall closure.
Here we would note the inclusion of “surgeon bias” (who performs laparotomy closure: a resident or a staff surgeon?) as the possible cause of the differences between van Ramshorst et al.’s1 results and ours. Although Webster et al.9 mentioned that participation from a 4th year resident as principal surgeon is a risk factor for abdominal wound dehiscence, a recent study by Kiran et al.,12 using a sample of over 60000 patients, concluded that resident participation in surgical procedures is safe. Although the variable “who performs the laparotomy closure?” was not used by van Ramshorst et al.1 or by us, the hospital where they carried out their study and our hospital are university hospitals where it is common for a resident to be present during surgery, therefore “surgeon bias”, if there is any, would exist in both studies and we would not consider it a reason for any difference between our results.
For preoperative prediction of abdominal wound dehiscence in our study we calculated the preoperative risk score (modified van Ramshorst). Although its application with the data from our sample showed statistically significant differences between the 2 subgroups, from a clinical viewpoint it would be difficult to decide whether or not a patient were at risk of abdominal wound dehiscence (overlapping of mean confidence intervals). Furthermore, the ROC curve of the preoperative score shows its lower predictive capacity, which may be explained by the important role surgical wound infection represents (a postoperative variable) as a risk variable. And finally, when we observe the sample distribution by preoperative risk categories, we can appreciate that the majority of patients with abdominal wound dehiscence are grouped together in a single category and that the calculation of probability of abdominal wound dehiscence with the equation also underestimates the real abdominal wound dehiscence percentage. We therefore believe that the preoperative risk score of abdominal wound dehiscence is of limited usefulness and it requires adjustment to improve results.
As stated above, for the preoperative abdominal wound dehiscence risk score to be efficient it requires adjustment. Its usefulness could be improved by using pre-calculated postoperative variables prior to surgery, for example: (1) if the patient has a history of chronic respiratory disease he or she could be given a risk value for postoperative cough, and similarly, (2) in the case of surgical infection, each patient could be given a risk value proportional to the risk of infection of each type of surgical wound.13 However, these empirical approximations would have to be tested and validated in future studies.
It is also important to mention that van Ramshorst et al.1 proposed that patients with an overall abdominal wound dehiscence risk score above 6 be given prophylactic management to prevent abdominal wound dehiscence. In our study, Table 3 shows that in the group with a global risk score of between 4 and 5.99, the mean probability of calculated dehiscence is 6.2% and the real percentage of abdominal wound dehiscence is 16%. Therefore, according to our results, those patients with a score of 4 or more in the global score would benefit from the use of prophylactic abdominal wound dehiscence management.
Following constructive criticism of the score developed by van Ramshorst et al.,1 and in the light of our results, we believe it is pertinent to point out the strengths and weaknesses of our own study.
We consider that the main strong point of our study is the use of the prospective database for adverse events from our department2,3 and our hospital's clinical computerized workstation. These resources have enabled us to obtain the exact and real data of almost all study variables, with the exception of postoperative cough. This prospective record of adverse events2,3 would partly explain why the incidence of abdominal wound dehiscence in our sample (8.5%) is above the value reported in literature (0.2%–6%),11,12,14 since the retrospective nature of the studies tend to report lower incidences.4
Other strengths of our work are: the inclusion of midline laparotomies alone, compared with the inclusion of all types of laparotomies4,5 in other studies; and that the percentage of emergency surgery represents almost half of our sample, since we know that this type of surgery is prone to a higher rate of abdominal wound dehiscence.1,4,9,12,13 These 2 factors also help to explain the higher incidence of abdominal wound dehiscence in our sample, compared with that described in published literature.
The following could be considered weak points in our study:
- (1)
Sample size: the abdominal wound dehiscence aetiology is multifactorial,6,7,10 resulting in clinically relevant percentage differences between the groups (gender, chronic pulmonary disease, emergency surgery, type of surgery) which could obtain statistical significance with a higher sample size.
- (2)
The percentage of postoperative cough in our sample is underestimated, due to the lack of a strict record of this variable in sources of used data.
- (3)
The low number of patients with ascites1,9,10 and jaundice,1,15 which are probable abdominal wound dehiscence risk factors, prevented us from reaching conclusions on the effect of these factors. This is because in our hospital hepatobiliary surgery is mainly performed by a subcostal incision.
- (4)
Similarly, few patients presented with anaemia,4,7,16 which could be connected to: (a) the strict values used in defining anaemia1; and also (b) the preoperative evaluation made by the Anaesthetics Department of our hospital, after which there are specific protocols to optimize haemoglobin levels prior to surgery.
- (5)
The majority of patients from the sample had undergone operations of the colon and small intestine. Although van Ramshorst et al.1 state that colonic surgery is the most frequent surgery in patients with abdominal wound dehiscence,1 in our study this result could have been overestimated due to the high percentage of patients who underwent colon surgery and the low presence of patients who underwent any other type of surgery. In our hospital this would be due to the preferred use of subcostal incisions when performing hepatobiliary and gastroduodenal surgery.
Finally, we can conclude that the usefulness of the risk model developed by van Ramshorst et al.1 to predict the risk of preoperative abdominal wound dehiscence, among patients undergoing midline laparotomy in the General and Digestive Surgery Department of Sabadell's Parc Tauli Hospital –Health and University Corporation, Barcelona is limited by the role played by the postoperative variables, mainly surgical wound infection. Use of the preoperative abdominal wound dehiscence risk score (modified van Ramshorst) requires adjustments in order to improve prognostic performance.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Gómez Díaz CJ, Rebasa Cladera P, Navarro Soto S, Hidalgo Rosas JM, Luna Aufroy A, Montmany Vioque S, et al. Validación de un modelo de riesgo de evisceración. Cir Esp. 2014;92:114–119.