The possibility of modelling diagnostic images in three dimensions (3D) in pancreatic surgery is a novelty that provides us multiple advantages. A better visualization of the structures allows us a more accurate planning of the surgical technique and makes it easier the surgery in complex cases.
We present the case study of a borderline pancreatic head adenocarcinoma patient to illustrate the advantages of 3D modelling in complex pancreatic surgery.
The help of 3D technology allowed us to optimally plan the intervention and facilitate surgical resection. The use of this tool could translate into: shorter operative time, fewer intraoperative complications or an increase in R0 resections. The usability of the program used in our case, agile and intuitive, was an added advantage.
La posibilidad de modelización de imágenes diagnósticas en tres dimensiones (3D) en cirugía pancreática es una novedad que nos aporta múltiples ventajas. Una mejor visualización de las estructuras nos permite una planificación de la técnica quirúrgica más precisa y nos facilita la realización de la cirugía en casos complejos.
Presentamos el caso de un paciente diagnosticado de un adenocarcinoma de cabeza de páncreas borderline para ilustrar las ventajas de la modelización 3D en cirugía pancreática compleja.
La ayuda de la tecnología 3D nos permitió planificar de manera óptima la intervención facilitando la resección quirúrgica. El uso de esta herramienta podría tRHAucirse en: menor tiempo operatorio, menores complicaciones intraoperatorias o un aumento de las resecciones R0. La usabilidad del programa utilizado en nuestro caso, ágil e intuitivo, fue una ventaja añadida.
Three-dimensional (3D) technology is quickly and firmly becoming established in the world of surgery due to the many advantages that it can provide when planning operations, facilitating vision in the operating room or improving teaching.
Over the last few years, several programs have appeared that allow us to obtain 3D images prior to surgery, especially in liver surgery. However, the development of 3D models for pancreatic surgery has not been as widespread.
The objective of this article is to present a new tool that can assist surgeons when planning and performing complex pancreatic surgery.
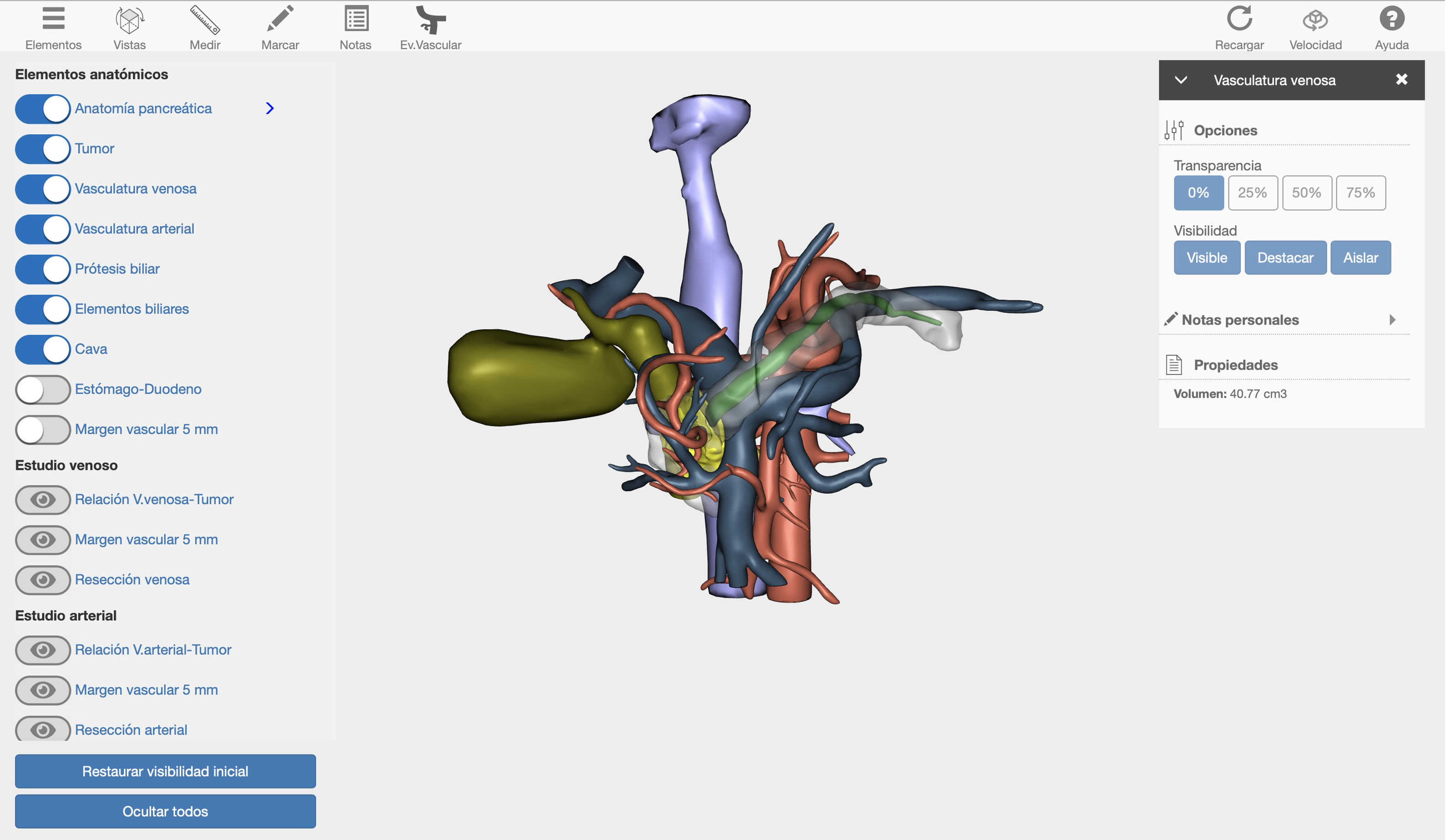
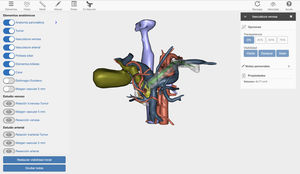
Surgical techniqueWe present the case of a 40-year-old male patient with borderline resectable pancreatic cancer. After discussion of the case in the multidisciplinary committee, we decided to place a coated metallic biliary stent and administer neoadjuvant chemotherapy treatment (FOLFIRINOX, 12 cycles). The computed tomography (CT) evaluation of the response to treatment revealed a neoplasm in the head of the pancreas, which was in contact with the mesenteric-portal venous axis in more than 180°. In addition, there was tumor contact with the superior mesenteric artery (SMA) and invasion of the right hepatic artery (RHA) originating at the SMA. Given the complexity of the case, a 3D image was created with the Cella Medical Solutions® Virtual Model, which confirmed the CT findings and also allowed us to obtain:
- -
360° visualization of the tumor and related adjacent structures (pancreatic and biliary anatomy, stomach, duodenum, portal venous system, vena cava and arterial vascularization) with the possibility to modify and adapt the parameters and movement of the image in 3D (Fig. 1).
- -
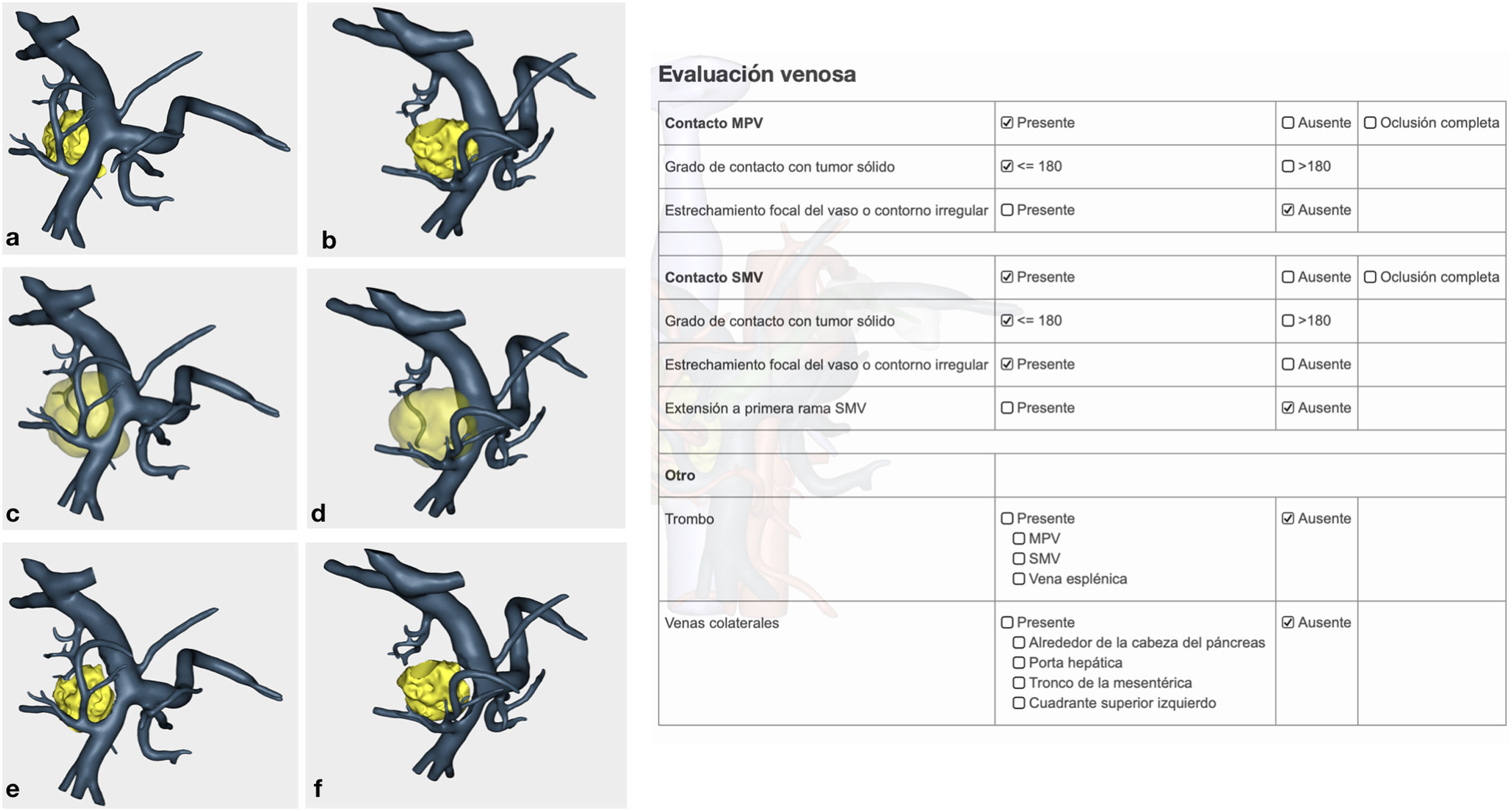
Venous study with the ability to observe a 360° image of the relationship between the tumor and the superior mesenteric vein (SMV), splenic vein, portal vein and their collateral branches. In addition, the program offers objective evaluation in accordance with NCCN1 criteria (Fig. 2).
- -
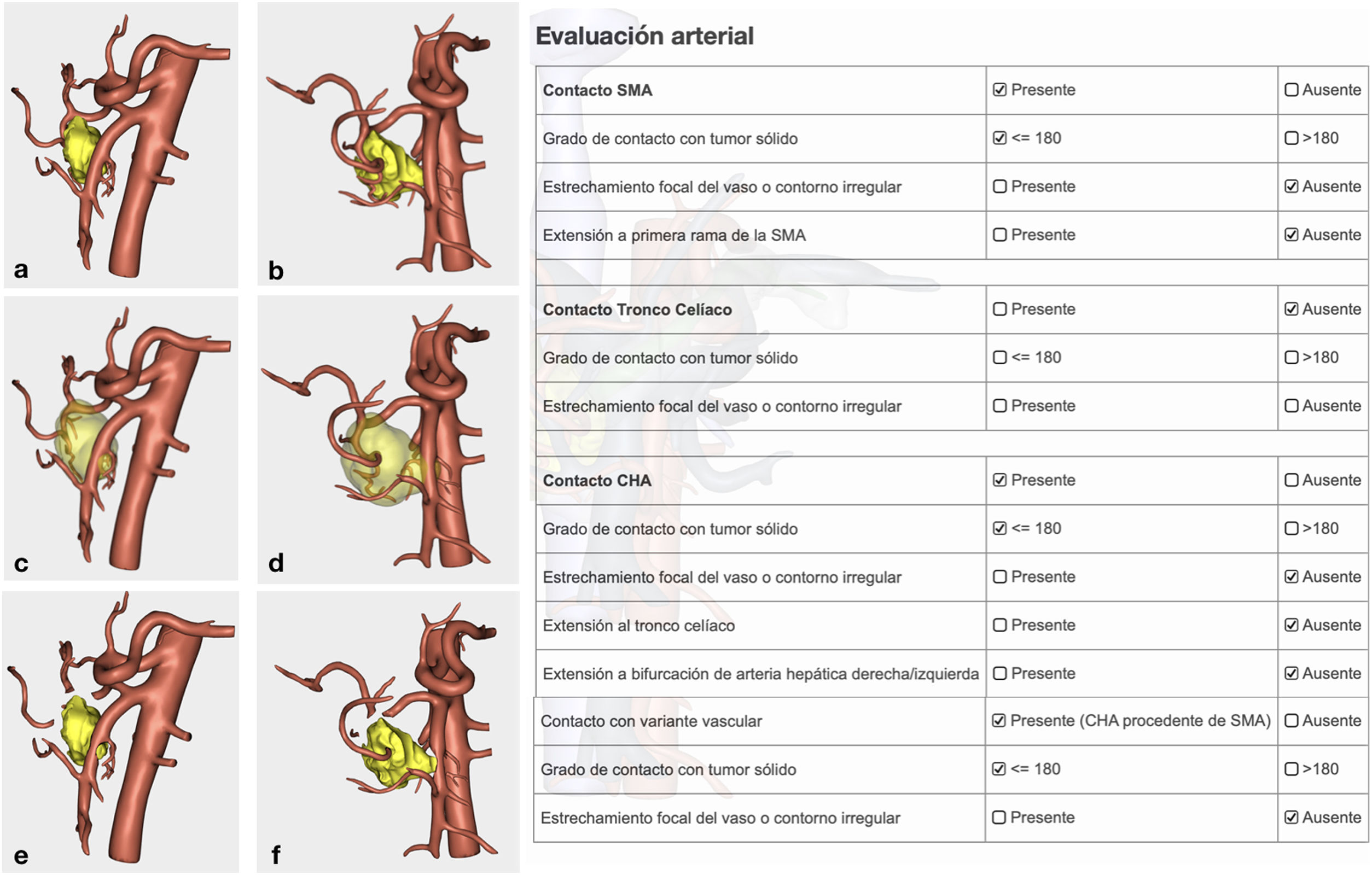
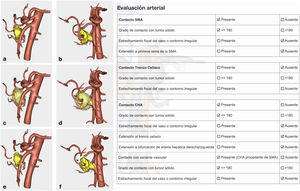
Arterial study with the ability to observe a 360° image of the relationship between the tumor and the most important arterial structures (SMA, celiac trunk and its collateral branches, and anatomical variants). Their objective evaluation also followed NCCN1 criteria (Fig. 3).
- -
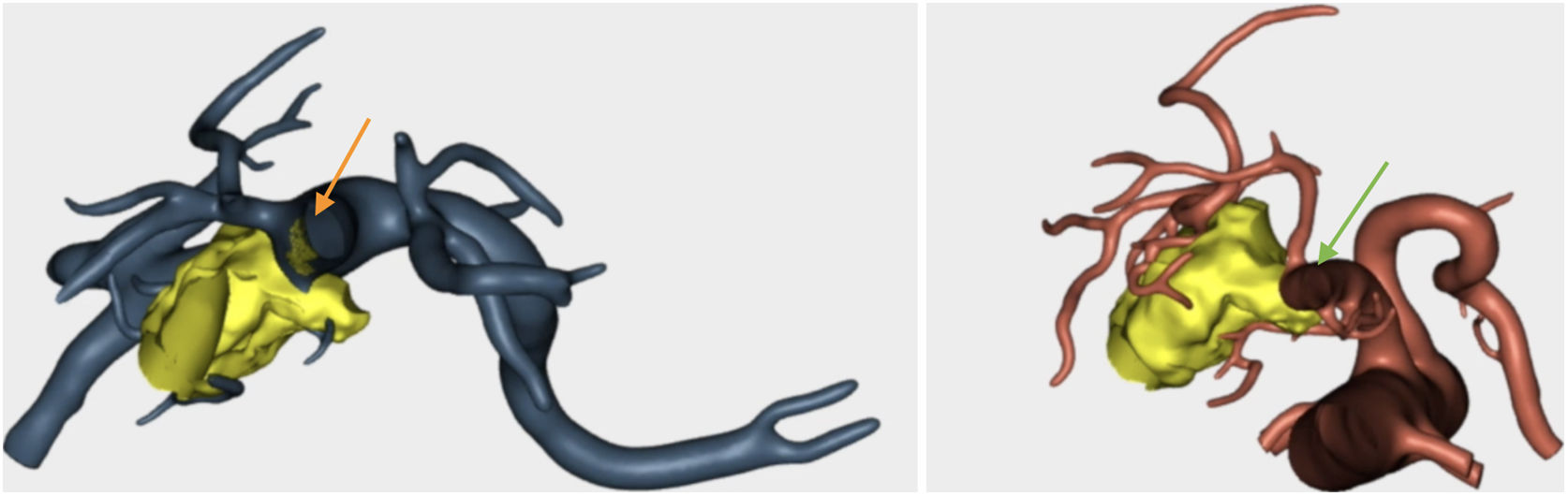
Confirmation of whether the tumor contact areas with the SMV, SMA and RHA were tumor areas with invasion of vascular structures or not (Fig. 4).
After the findings provided by the complementary tests, and in the absence of disease progression during preoperative therapy, we decided to proceed with exploration in the operating room. Using bilateral subcostal laparotomy, we carried out a pancreaticoduodenectomy (PD). As an RHA originating at the SMA had been identified during the preoperative study, we performed a resection using the initial SMA approach. The highlights of the surgery were:
- -
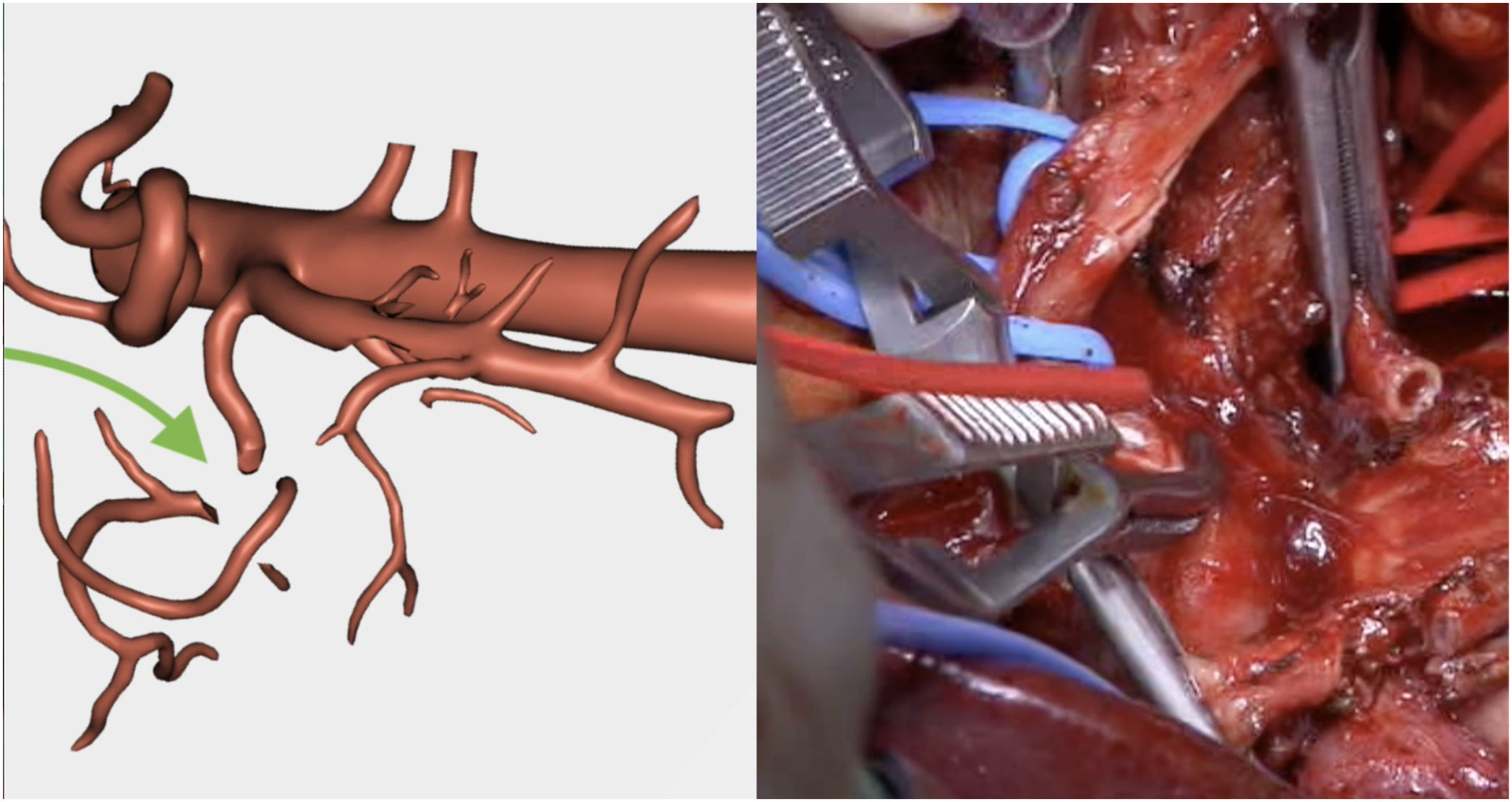
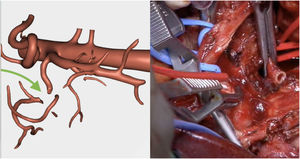
Resection of the RHA (Fig. 5) and end-to-end anastomosis
- -
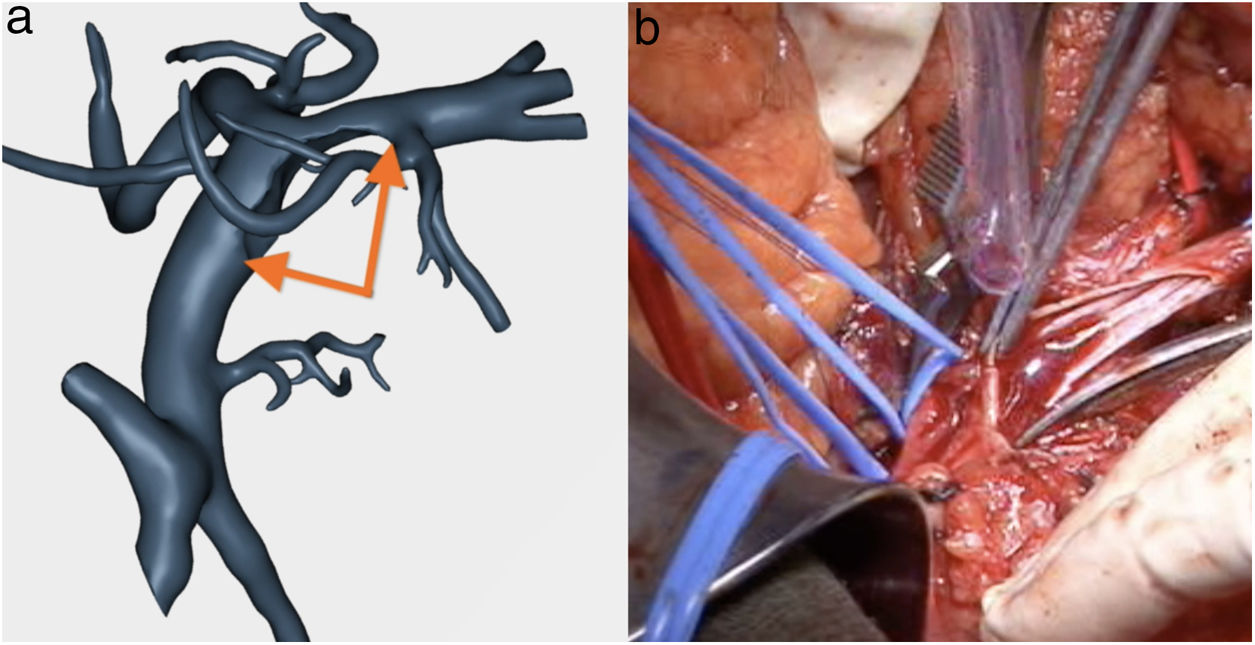
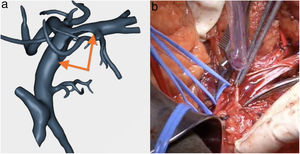
SMV resection (Fig. 6) and Clavien plasty repair
- -
At the area of contact with the SMA, we were able to dissect the tumor without resecting the artery as there was no invasion.
The technique chosen for the reconstruction included an invaginating pancreatic–gastric anastomosis, end-to-side hepaticojejunal anastomosis, manual antecolic end-to-side gastrojejunal anastomosis, and construction of a Braun omega loop. Two non-suction drains were placed, one in the right hypochondrium and the other adjacent to the pancreatic-gastric anastomosis.
The postoperative period was uneventful. The drain tube was removed from the right side 48 h after surgery. Amylase was analyzed 72 h after the procedure in the discharged fluid of the left drain, which was withdrawn as the result was less than 3 times the amylase levels in blood.
There were no 30-day or 90-day postoperative complications.
The pathology study reported a moderately differentiated ductal adenocarcinoma (pT3N2 Mx) with a medial resection margin (venous) at 1 mm, and the remaining margins were free. This confirmed the tumor involvement of the SMV and the RHA and no invasion of the SMA. Therefore, it coincided with the preoperative diagnosis made by the 3D model (Fig. 4).
We have currently used this 3D reconstruction program for pancreatic surgery in 12 patients. The indication used in our hospital is: patients with borderline pancreatic cancer, in whom resectability is uncertain. In these 12 cases, the 3D model has helped us to:
- -
determine the division points of the SMV and plan its reconstruction in advance in 10 patients;
- -
diagnose uncommon anatomical variants that are difficult to interpret on CT scan (RHA and left as independent branches of the celiac trunk and gastro-duodenal artery as terminal branch of the left hepatic artery) in the context of a locally advanced tumor in the head of the pancreas, and other more common ones such as RHA originating at the SMA;
- -
confirm the diagnosis of non-resectability due to the impossibility to reconstruct the SMV in 2 cases;
- -
open an alternative for tumor resection when it appeared unresectable on CT in one case; and
- -
as a teaching tool in all cases.
The use of 3D images constructed from CT scans or magnetic resonance imaging (MRI) is widespread in other surgical procedures (liver resections, for instance), but they are used less often for pancreatic pathologies. These diagnostic tests are currently gaining popularity because they have been proven to be very useful in surgical planning, especially for surgeons with less experience.2
The possibility of creating 3D reconstructions for a structure like the pancreas, which is a significant surgical challenge, opens up future expectations towards 3D visualization in the surgical field itself.3 The 3D images are made by processing the information from the patient’s preoperative tests (CT, MRI, PET, etc). This diagnostic tool provides: more complete and simpler planning of the resection to be carried out; easy identification of vascular anatomical variants (in our case an RHA from the SMA); the ability to discuss cases online with other surgeons who are not in the same place; and, more effective teaching.
Although the current indication for using the 3D program in our hospital is borderline resectable pancreatic cancer, these indications could be extended. We believe that the information provided is very valuable for performing resections with greater safety, which could be reflected in shorter surgical times and even reduce complications or R1 resections. Unresectable patients could also be more precisely identified, thereby reducing the number of exploratory laparotomies or assisting with therapeutic decision-making. Although the agreement between the 3D study and the surgery has exceeded our expectations, the eventual advantages of this new tool should be evaluated in a study designed for this purpose.
One of the potential drawbacks of this type of diagnostic reconstruction is its cost. However, we can find 3D reconstructions with a price no higher than that of other consumable materials commonly used in this type of surgery. Therefore, using this tool does not seem to be a disadvantage when we consider that this cost is largely outweighed by the advantages described above (avoiding exploratory laparotomies in unresectable patients, reducing complications, etc). Another problem we face with these programs is usability. However, the increasingly precise development of these tools goes hand in hand with the simplification of their use to make them agile and intuitive. In this case, the program used is designed to provide the information that surgeons would like to obtain before facing a case of these characteristics in a simple manner, with no need for prior training in it use. In addition, the images can be consulted from different devices (computer, mobile phone, operating room monitors, etc), which facilitates their access. There is also the possibility of printing a 3-dimensional model when appropriate. In our case, we have only considered it once, for educational purposes.
Several studies have been published about the use of 3D images in surgery. Despite this, their development in pancreatic surgery has not been as extensive. Three-dimensional images have been used statically to study volumes, locate structures,4,5 or to plan specific surgeries.6 Until now, no other dynamic program with the possibility of 360° visualization had been developed that is so easy for the surgeon to use, providing a view of the relationship between the different structures and precise identification of the tumor, thereby simplifying appropriate planning of the surgical technique for each patient.
Conflict of interestsThe INCLIVA Biomedical Research Institute has collaborated in the development of the 3D modelling program for pancreatic surgery (Cella Medical Solutions®).
We would like to thank Raúl Sales for his collaboration in recording images of the surgical field.
Please cite this article as: Garcés-Albir M, Muñoz-Forner E, Dorcaratto D, Sabater L. ¿Qué aporta la imagen tridimensional preoperatoria en la cirugía pancreática compleja? Cir Esp. 2021;99:602–607.