The most common cause of pleural empyema are parapneumonic effusions, and lung cancer is a rare cause of empyema. The aim of the present study is to analyse the results of the thoracoscopic treatment of empyema before definitive oncological treatment.
MethodsRetrospective descriptive study of 332 patients including different clinical variables between 2002 and 2010.
ResultsAmong 332 patients with empyema, the etiology of this disease was lung cancer in 11 patients. Ten of these patients were male and one was female (median age, 57.9 years; range, 46–76). The initial treatment was tube thoracostomy in 8 patients and video-assisted thoracoscopic surgery in 3 patients. Thoracoscopic debridement was performed in 4 patients whose tube thoracostomy underperformed because of insufficient drainage. The methods used for diagnosis of lung cancer were fiberoptic bronchoscopy and video-assisted thoracoscopic surgery. Surgical resection was performed on 7 suitable patients following infection control. Postoperative bronchopleural fístula and empyema occurred after pneumonectomy in one case. No operative mortality was observed. The mean survival time was 32.8 months for patients undergoing resection.
ConclusionsEmpyema could be a rare presentation of lung cancer and those suitable for surgical treatment should undergo standard treatment with reasonable results.
La causa más frecuente de los empiemas pleurales son los derrames paraneumónicos, siendo el cáncer de pulmón el origen de los mismos en muy pocas ocasiones. El objetivo de nuestro trabajo es analizar los resultados del tratamiento del empiema por vía toracoscópica previo al tratamiento oncológico definitivo.
MétodosEstudio descriptivo retrospectivo en el que se recogen las diferentes variables clínicas durante el tratamiento de 332 pacientes entre 2002 y 2010.
ResultadosEn 332 pacientes con empiema, la etiología de esta enfermedad fue el cáncer de pulmón en 11 casos. Entre ellos había 10 varones y una mujer (mediana de edad, 57,9 años; rango: 46-76). El tratamiento inicial fue la colocación de un tubo de toracostomía en 8 pacientes y la cirugía toracoscópica videoasistida en 3 pacientes. Se llevó a cabo un desbridamiento toracoscópico en 4 pacientes en los que el resultado del tubo de toracostomía no fue satisfactorio debido a un drenaje insuficiente. Se llevó a cabo una resección quirúrgica en 7 pacientes cuyas características eran apropiadas tras el control de la infección. Se produjo una fístula broncopleural y un empiema postoperatorio tras la neumectomía en un caso. No se observó mortalidad operatoria. La media de supervivencia fue de 32,8 meses en los pacientes tratados con resección.
ConclusionesEl empiema puede ser una forma de presentación muy poco frecuente del cáncer de pulmón cuyo abordaje puede realizarse por toracoscopia, si bien el mismo tiene sus limitaciones.
Empyema is defined as a collection of pus in the pleural cavity. Empyema remains a potentially serious condition with multiple causes. Although it most commonly occurs after parapneumonic effusion, it also occurs as a complication of lung cancer in 0.1%–7.9% of cases of empyema and in 0.7% of patients with operable primary lung cancer.1,2
Empyema can be controlled by drainage of the thoracic cavity and systemic treatment with antibiotics. Drainage is achieved with tube thoracostomy or video-assisted thoracoscopic surgery (VATS). If lung cancer is the cause of the empyema, it should be staged, and if the cancer stage is suitable, lung resection can be performed.
As only a limited of number of cases are reported in the literature, we sought to evaluate the treatment of empyema in primary lung cancer with resectable tumors.
Materials – MethodsUsing our hospital's database, we retrospectively investigated 332 patients who were diagnosed with empyema between 2002 and 2010. All of the patients who were treated in our clinic provided informed consent by signing a statement allowing the use of their data for clinical trials. Patients were classified according to etiology, which was determined based on the patient's history; findings of physical examination, radiology, and empyema fluid analysis; and other clinically relevant factors. Postoperative, post-traumatic, post-pneumonic empyema, and metastatic lung cancer patients were not included in this study. Our study included patients with a diagnosis of primary lung cancer during the treatment of empyema. Data collected included demographics, subtype of pulmonary tumor, diagnosis, staging, surgical procedures, outcomes, and follow up.
Complete blood counts, biochemical parameters, and sputum tests for tuberculosis and non-tuberculosis were evaluated in all patients. Imaging studies (e.g., posteroanterior and lateral chest radiography, ultrasonography, thoracic computerized tomography (CT), and positron emission tomography) were also performed. Thoracic CT was used to determine parenchymal lesions of lung.
Thoracentesis was performed in all cases. Pleural fluid was sent for biochemical, microbiological, and pathological examination. Empyema was defined as culture-positive pleural fluid, purulent appearance, smell, and biochemical parameters. All patients with a suspected pleural infection received appropriate antibiotic treatment from the time of first review.
Tube thoracostomy and/or VATS were performed first, based on the physical status of the patient, for drainage of empyema. If drainage was insufficient following tube thoracostomy in a patient, VATS was also performed as a secondary procedure.
Single lung ventilation was achieved using a double lumen endotracheal tube at the time of VATS. Fibrin deposits were cleaned from the parenchyma of the lung, ribs, and diaphragm, and the thoracic space was washed with saline during the operation. After lung was freed from the chest wall and diaphragm, lung capacity was checked by inflating the lung to fill the pleural cavity. We obtained biopsies from the parietal pleura and the parenchyma for verification of histopathology. A 32-French drain was inserted into the thorax for drainage at the end of the operation. All patients were extubated in the operation room after VATS and they were transferred to a postoperative recovery room. Criteria for the drain endpoint included absence of air leaks, full lung expansion on a chest radiograph, and 50cc/day or lower sterile serous drainage.
Fiberoptic bronchoscopy was performed if no diagnosis was obtained with VATS for a lung lesion observed by CT. Patients diagnosed with primary lung cancer were staged following treatment of their empyema.
Pulmonary resection and mediastinal lymph node dissection by thoracotomy were performed in selected patients. If surgery was unsuitable, the patient was redirected to the department of medical oncology.
ResultsOf the eleven patients in this study, ten were men and one was a woman; all were diagnosed with primary lung cancer with empyema. The age range was 46–76 years (mean, 57.9 years). The most frequent complaints were fever, dyspnea, and chest pain. A decrease in breath sounds in the affected hemithorax was a prominent finding upon physical examination. Mycobacterium tuberculosis was not detected in sputum and pleural fluid of any patients.
Tube thoracostomy was performed as a first procedure in eight patients with high fever, dyspnea, and leukocytosis. Four of them underwent VATS due to insufficient drainage. In order to save time, VATS was the first surgical approach in three clinically stable patients with loculation observed on CT and ultrasonography. The mean drainage time was 7.63 (range, 3–14) days.
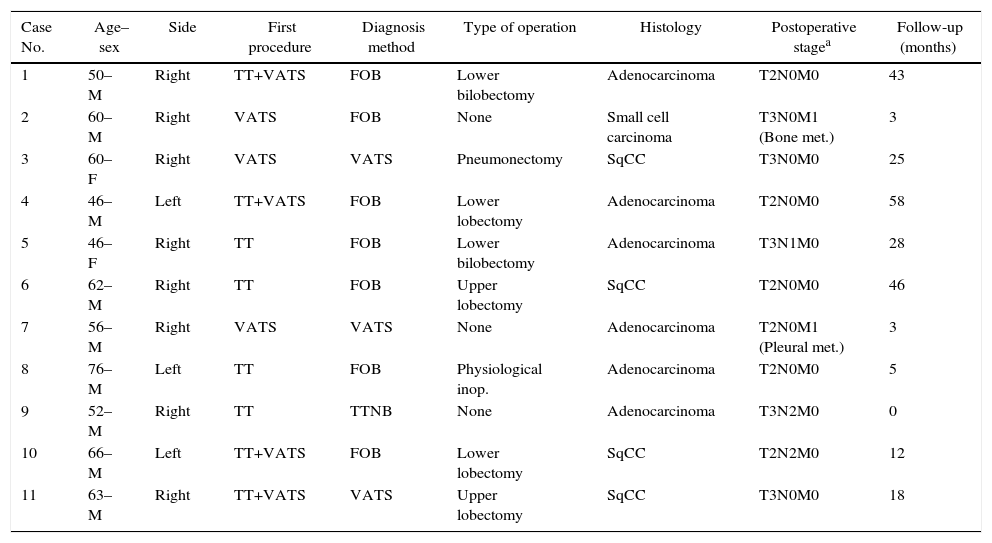
The diagnostic procedure for primary lung cancer included fiberoptic bronchoscopy in seven patients, trans-thoracic fine needle aspiration biopsy in one, and VATS in three patients. Histopathological results included small cell carcinoma in one patient, adenocarcinoma in 6, and squamous cell carcinoma in 4 cases. Table 1 shows patients’ demographical and clinical backgrounds. Cytological examination revealed no malignant cells in patients who underwent thoracostomy.
Demographic and Clinical Features of Patients.
| Case No. | Age–sex | Side | First procedure | Diagnosis method | Type of operation | Histology | Postoperative stagea | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50–M | Right | TT+VATS | FOB | Lower bilobectomy | Adenocarcinoma | T2N0M0 | 43 |
| 2 | 60–M | Right | VATS | FOB | None | Small cell carcinoma | T3N0M1 (Bone met.) | 3 |
| 3 | 60–F | Right | VATS | VATS | Pneumonectomy | SqCC | T3N0M0 | 25 |
| 4 | 46–M | Left | TT+VATS | FOB | Lower lobectomy | Adenocarcinoma | T2N0M0 | 58 |
| 5 | 46–F | Right | TT | FOB | Lower bilobectomy | Adenocarcinoma | T3N1M0 | 28 |
| 6 | 62–M | Right | TT | FOB | Upper lobectomy | SqCC | T2N0M0 | 46 |
| 7 | 56–M | Right | VATS | VATS | None | Adenocarcinoma | T2N0M1 (Pleural met.) | 3 |
| 8 | 76–M | Left | TT | FOB | Physiological inop. | Adenocarcinoma | T2N0M0 | 5 |
| 9 | 52–M | Right | TT | TTNB | None | Adenocarcinoma | T3N2M0 | 0 |
| 10 | 66–M | Left | TT+VATS | FOB | Lower lobectomy | SqCC | T2N2M0 | 12 |
| 11 | 63–M | Right | TT+VATS | VATS | Upper lobectomy | SqCC | T3N0M0 | 18 |
F, female; FOB, fiber-optic-bronchoscopy; Inop., inoperable; M, male; Met., metastasis; SqCC, squamous cell carcinoma; TT, tube thoracostomy; TTNB, trans-thoracic needle biopsy; VATS, video-assisted thoracoscopic surgery.
Positron emission tomography (PET) was performed on only three patients, as PET was not available to other patients. This scan was useful for the detection of distant tissue metastases in the infective state, but not for local evaluation because of the high false-positive rate.
Mediastinoscopy was performed in six patients who had surgically resectable tumors. The types of resection were pneumonectomy in one patient, lower lobectomy in two, lower bilobectomy in two, and upper lobectomy in two patients. Severe inflammatory adhesions were noted in patients who underwent right pneumonectomy. The bronchial stump was supported with intercostal muscle flap, mediastinal fatty tissue, or azygos vein in all patients. Among postoperative complications, one bronchopleural fistula and empyema occurred in a patient who underwent pneumonectomy on the 23rd postoperative day, and supra-ventricular tachycardia was observed in a patient who underwent lower bilobectomy. We performed open drainage using an Eloesser flap on the patient with bronchopleural fistula.
Four patients could not undergo surgical treatment because of metastasis (bone, pleural) and physiological inoperability. The time between hospitalization and diagnosis of lung cancer ranged from 13 to 24 days.
We did not wait to perform thoracotomy in patients with resectable tumors after treating the empyema; lung resection was performed as a follow-up procedure for immediate eradication of the infection. Our patients’ follow up time was between 1 and 58 months. Survival time was 12–58 months (mean, 32.8 months) in the surgical group and between three and five months in the non-surgical group (Table 1).
DiscussionPleural empyema is a serious problem that affects all age groups; however, there is currently no standard approach for the treatment of this disease. The etiology of empyema includes parapneumonic effusions, surgical procedures, thoracic trauma, perforation of the esophagus, sub-diaphragmatic infection, and malignancy.
Symptoms of a patient with empyema may include fever, tachypnea, and an increasing oxygen requirement. Imaging is very important for diagnosis and management of pleural empyema. Thoracic CT with intravenous contrast can be used to differentiate between parenchymal and pleural processes, identifying pleural thickening and loculations and masses. PET/CT is an efficient tool for whole-body scanning, but pulmonary infection may cause false-positive findings in patients with lung cancer.3 We determined bone metastasis in one patient by PET/CT.
Multiple approaches exist for treating parapneumonic effusions, ranging from antibiotics to radical surgical intervention. Methods to drain parapneumonic effusion include thoracentesis, tube thoracostomy alone, tube thoracostomy with chemical/enzymatic debridement, and VATS debridement.
Currently, the minimally invasive VATS approach is the gold standard for operative management of fibrinopurulent pleural space disease.4–6 We recommend debridement by VATS to avoid pleural thickness. VATS is useful to determinate lung cancer (three patients in the study), pleural implant from malignancy (one patient in the study) and evaluate mediastinal invasion of the tumor. VATS is also easily performed by a single port access.7
After the mass/nodule was seen in the lung, histopathological verification may be done by fiber-optic-broncoscopy. We saw an endobronchial lesion in 7 patients in this study. Trans- thoracic needle biopsy was used to evaluate malignancy in one patient. Staging of lung cancer was done by CT, PET-CT and mediastinoscopy in our study. Interval in days between the first evaluation and staging was 7–23 days in a Spanish lung cancer screening program.8 The time was 13–24 days in the article.
The possible mechanism of pleural infection in patients with concomitant lung cancer and empyema may include: (1) infection of the pleural space as a result of repetitive thoracentesis in patients with pleurisy (iatrogenic); (2) occurrence of a broncopleural fistula because of rupture of a cavitary carcinoma (as part of the natural progress of malignancy); (3) obstruction of the bronchus by a mass resulting in pneumonia and atelectasis (due to localization of the cancer); and (4) empyema may occur by tumor necrosis after chemotherapy. In this study, empyema occurred secondary to atelectasis in the first, second, and sixth cases; bronchopleural fistula in the fifth case; and an iatrogenic cause in the third and fourth cases. We could not determine the cause of the empyema in the other cases. Some authors indicated that empyema can occur after transbronchial lung biopsy.1 Immunosuppression has also been suggested as a cause for pleural infection.2
When the patient's general condition improved, elective surgery was performed; surgical resection during severe thoracic infection carries too high a risk of complications.
Upon review of the literature, we determined that 25 patients who had concomitant lung cancer and empyema underwent pulmonary resection.1,2,9–12 Tube thoracostomy was the most commonly used method for the treatment of empyema. Subotic and colleagues reported treatment of empyema in four patients before pneumonectomy.9 They diagnosed per-operative empyema in one of these cases, and they mentioned that surgical treatment could not be excluded because of pleural infection in patients with bronchial carcinoma. Fifteen patients were simultaneously treated for empyema and lung cancer in Riquet's study; in this study, fibrinolytic treatment was performed to treat an infection of the pleural space. Seven of them were treated conservatively, and nine underwent surgical procedures such as pneumonectomy (eight cases) and lobectomy (one case).2
In a study from Japan, the authors reviewed and discussed seven cases of concomitant empyema and lung cancer in patients who underwent pulmonary resection. The time between drainage and surgery ranged from 0 to 39 days in the reported cases.1 In our study, the mean time period was seven (range, 9–18 days) days.
We suggest that pneumonectomy should be avoided because of the risk of bronchopleural fistula. If pneumonectomy is necessary, a bronchial stump can be supported with mediastinal fatty tissue, omentum, azygos vein, intercostal muscle, or other muscles.
In conclusion, concomitant empyema and resectable lung cancer are a challenging situation. Primary lung tumors can be overlooked during the treatment of pleural infection, as empyema and lung cancer are very rarely seen together. Treatment of empyema should be performed as soon as possible, and the underlying cause of the inflammation should be identified. Malignancy should be kept in mind as a potential etiological factor in cases of empyema. The stage of lung cancer should be determined, and resection should be performed in selected patients after the treatment of their empyema. Drainage of the pleural space and sterilization must be performed thoroughly to lower postoperative complications after pulmonary resection.
Conflict of InterestNone.
Please cite this article as: Eryigit H, Orki A, Unaldi M, Ozdemir A, Orki T, Kosar A, et al. Tratamiento acelerado del empiema y cáncer de pulmón concomitantes mediante cirugía toracoscópica videoasistida. Cir Esp. 2016;94:100–104.