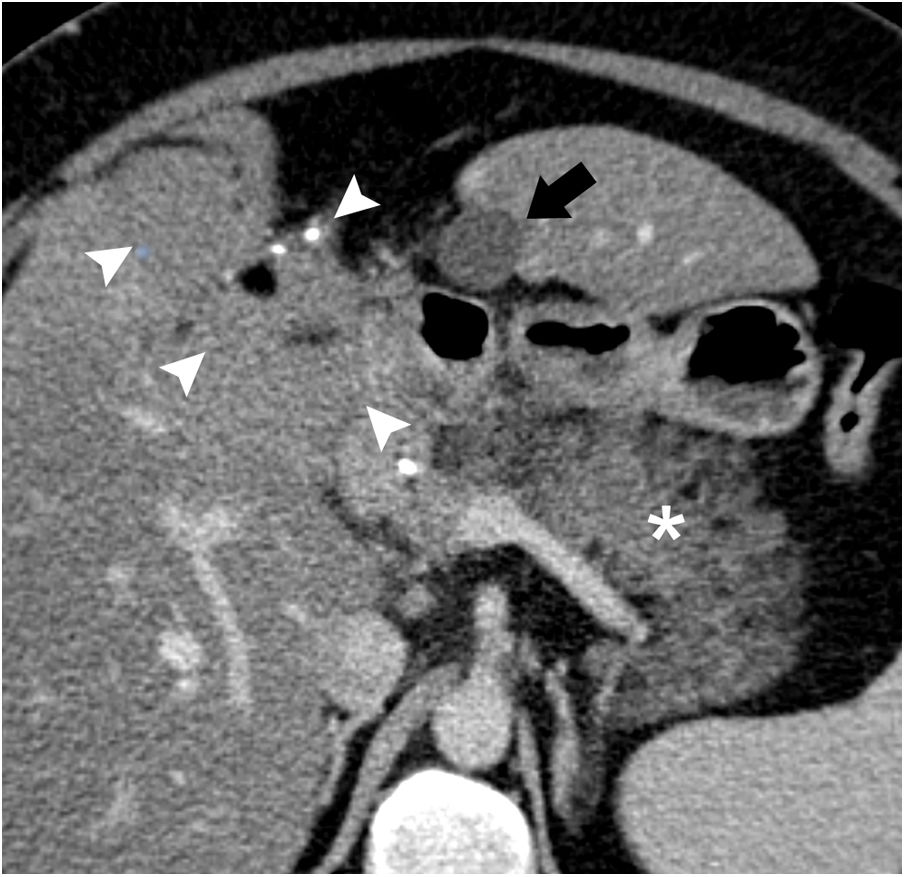
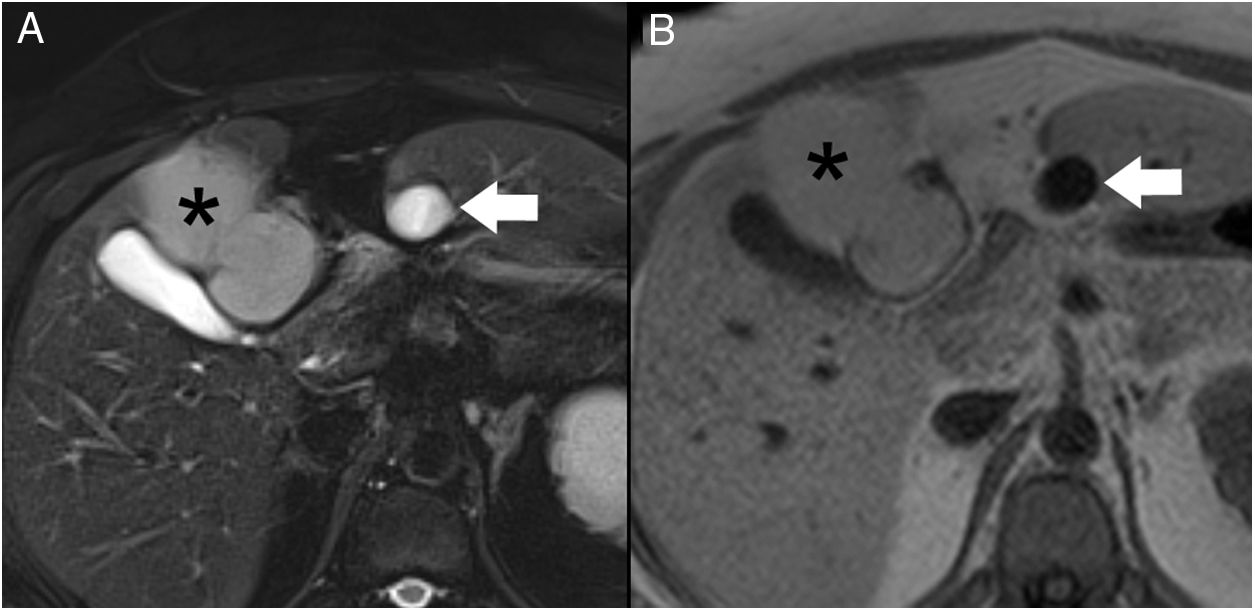
We present the case of a 40-year-old woman with renal hypoplasia, a smoker of 10 cigarettes a day, who was being treated with oral contraceptives. She had been previously admitted for an episode of mild pancreatitis, whose etiology could not be determined during hospitalization. A computed tomography scan with intravenous contrast (Fig. 1) demonstrated an edematous pancreas without necrosis. In addition, a subhepatic collection measuring 1.5 cm in diameter was identified with homogeneous liquid content, spindle-like morphology and thin walls. Incidentally, a liver lesion suggestive of a teratoma was observed. The patient was discharged for outpatient study. A liver MRI scan (Fig. 2) confirmed the cystic nature of the subhepatic lesion, showing notable hypointensity on T1 and hyperintensity on T2. At its cranial end, a tubular structure was identified measuring 3 mm in diameter, with the same signal intensity and directed towards the pancreas.
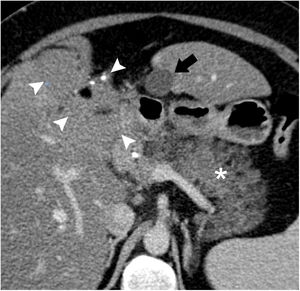
Axial view of computed tomography with iv contrast. A fusiform subhepatic collection is observed in contact with liver segment III (arrow); the content is homogenous, with liquid density and thin walls. The pancreas is edematous and increased in size (asterisk). A teratoma of the liver is observed (arrowheads) with calcifications and areas of fat attenuation.
The patient was admitted again due to epigastric abdominal pain radiating towards the back, accompanied by nausea and vomiting. She had no fever or jaundice. Lab work-up showed amylase 2936 U/L and lipase 9519 U/L, and the patient was diagnosed with acute pancreatitis.
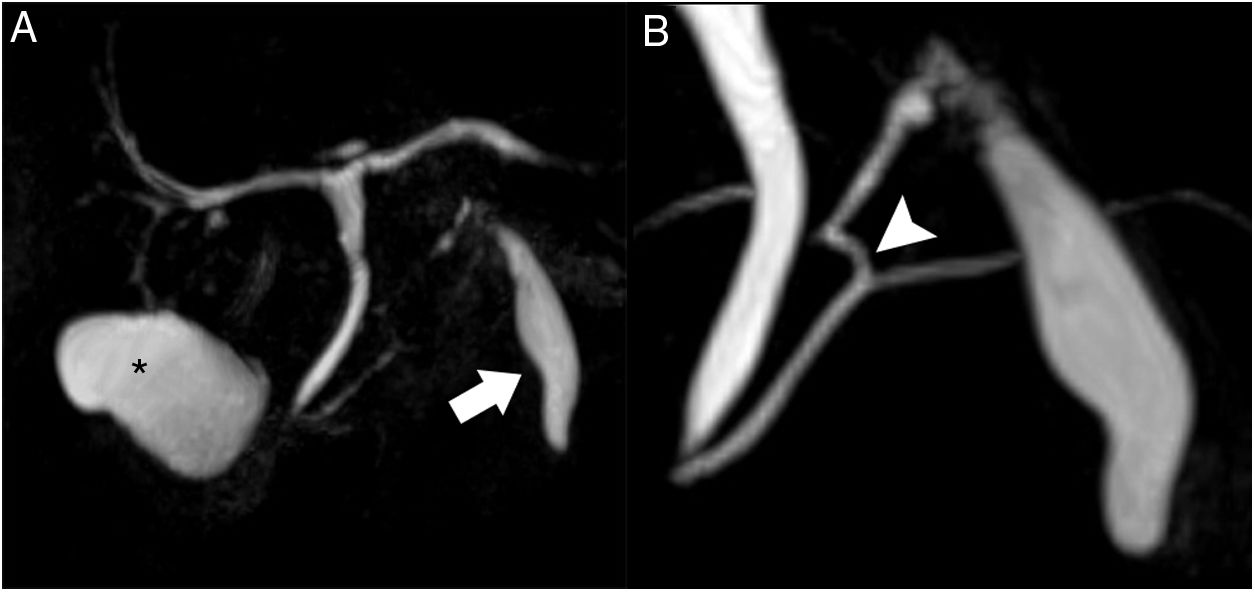
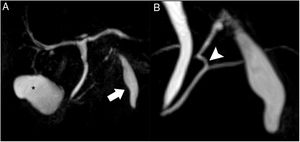
Magnetic resonance cholangiopancreatography (Fig. 3) showed a normal gallbladder with no lithiasis. The subhepatic collection remained unchanged, seen as a structure measuring 5 × 1.5 cm adjacent to liver segment III, with thin, regular walls. A duct was confirmed at its cranial end, measuring 3 mm in diameter, with a spiroid pathway in its first 2 cm, which communicated with the main pancreatic duct at the junction of its distal two-thirds. Based on these findings, the diagnosis was accessory gallbladder communicating with the Wirsung duct.
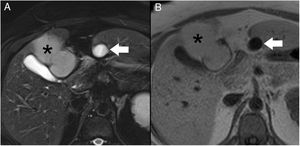
Magnetic resonance cholangiopancreatography. T2 TSE, volumetric reconstruction (A): the normal gallbladder is observed in its characteristic location (asterisk). Magnified view (B): the subhepatic collection shows an appearance similar to a gallbladder, located anterior to the pancreas (arrow); at the cranial end, a duct is observed that is joined to the main pancreatic duct at the junction of its distal two-thirds (arrowhead).
Subsequently, endoscopic ultrasound showed cholelithiasis in the gallbladder. Given the patient’s symptoms and the findings of the complementary tests, surgical intervention was indicated. During the operation, a cystic tumor measuring 15 cm in diameter was observed, which seemed to depend on the hepatic hilum but was only intimately adhered to the liver. In addition, a gallbladder of normal characteristics was evident, as well as a structure that simulated a gallbladder in contact with liver segment III, with a duct that entered into the pancreas. During surgery, intraoperative cholangiography confirmed that this structure drained into the pancreatic duct. In addition, amylase and lipase determinations of the liquid inside obtained values higher than 90,000 U/mL. We conducted simple excision of the cystic tumor, cholecystectomy and cholecystectomy of the gallbladder connected to the pancreas with ligation of the duct flush to its entry into the gland.
The pathology results reported that the mass labeled as hepatic was dependent on the lesser omentum; it also confirmed the diagnosis of teratoma. Macroscopically, the accessory gallbladder had a saccular morphology, showing a smooth serous surface that was grayish in color. After opening, it revealed a clear serous liquid, and the mucosa was whitish in color. Histologically, the wall was indistinguishable from that of the gallbladder.
The first observation of a pancreatic bladder was made by De Graaf in cats in 1664, involving a double gallbladder, one of which was pancreatic. In 1925, Boyden found 5 cases of gallbladder duplication in cats, where one of them communicated with the pancreatic canal, presented a similar histology. The first case of pancreatic bladder in humans was published in 1971, by Wrenn and Favara. It was a cystic structure with histology similar to the duodenum adjacent to the gallbladder, each one had a cystic duct that drained into the common hepatic duct through a common channel. Both the authors and Boyden, through a comment in the article, labeled the bladder as ‘pancreatic’ given its hypothetical embryological origin, despite not communicating with the pancreatic duct. They postulated that it was an aberrant growth of the ventral pancreas that, instead of turning around the duodenum, invaded the porta hepatis, forming a pancreatic bladder.1
There are 3 cases in the literature of single gallbladders with abnormal drainage to the pancreatic duct. These can be considered ‘pancreatic gallbladders’ based on their anatomical relationship with the gland. In the first, described by Atlas and Jacquemin in 1972,2 a bile duct was observed that originated in the right anterior hepatic sector that received the cystic duct and drained into the main pancreatic duct. The other 2 cases were described by Piel-Desruisseaux et al.3 in 1999 and consisted of normally located gallbladders that drained into the Wirsung duct. In addition, there are other cases of cystic structures with heterotopic tissue connected to the Wirsung duct. In 1971, Williams and Hendren described a duodenal duplication adjacent to the head of the pancreas,4 and in 1972 Akers et al. reported a cyst lined with pyloric mucosa adjacent to the body.5 In 1958 Bradbeer published the case of a saccular structure adjacent to the head of the pancreas connected to the pancreatic duct.6 The histology confirmed hypertrophic gastric mucosa; however, the author did not consider it a gastric duplication, but instead a diverticulum of the main pancreatic duct when not in contact with the stomach or communicating with it.
After a thorough review of the literature, we have only found one case of an accessory gallbladder communicating with the main pancreatic duct. In 1999, Ishibashi et al.7 described 2 adjacent gallbladders: one in the usual location adjacent to the liver, connected normally with the common hepatic duct through the cystic duct; and the second was adhered to it with drainage to the Wirsung duct through its own cystic duct. Ishibashi et al. stated that their case could be explained as either a pancreatic bladder, as it was separate from the bile duct and draining into the Wirsung, or as an accessory gallbladder with abnormal drainage to the pancreatic duct.7
Our patient presented 2 gallbladders. One had the typical morphology, location and histology of a normal gallbladder and was connected to the common hepatic duct. The other was located on the midline, posterior to liver segment III and in intimate contact with it. It was an oval structure with the major axis oriented in the craniocaudal direction, with a morphology reminiscent of a normal gallbladder with an identifiable fundus and body. At its cranial end, a duct began whose first 2 cm had a spiroid morphology. Then the path continued caudally and lateral right at a 90° angle, demonstrating smooth morphology to its distal end. At a distance of 7 mm from its junction with the dorsal portion of the main pancreatic duct, there was another 90° angle. This morphology could explain the abnormal duct junction with a secondary pancreatic branch.
As in the Ishibashi publication, our case could be considered a pancreatic bladder or an accessory gallbladder communicating with the pancreas. Given the location of the accessory gallbladder and connection of its duct to the dorsal pancreas, we do not believe that the embryological explanation provided by Boyden in the case of Wrenn and Favara or the term ‘pancreatic bladder’ can be applied for this reason.
In contrast with the cases of accessory structures draining to the pancreatic duct mentioned above, the morphology of the accessory gallbladder and its duct is practically identical to those found in the gallbladder and normal cystic duct. In addition, the histology is indistinguishable from a normal gallbladder. It therefore seems more appropriate to classify the structure as an accessory gallbladder with anomalous drainage to the Wirsung duct.
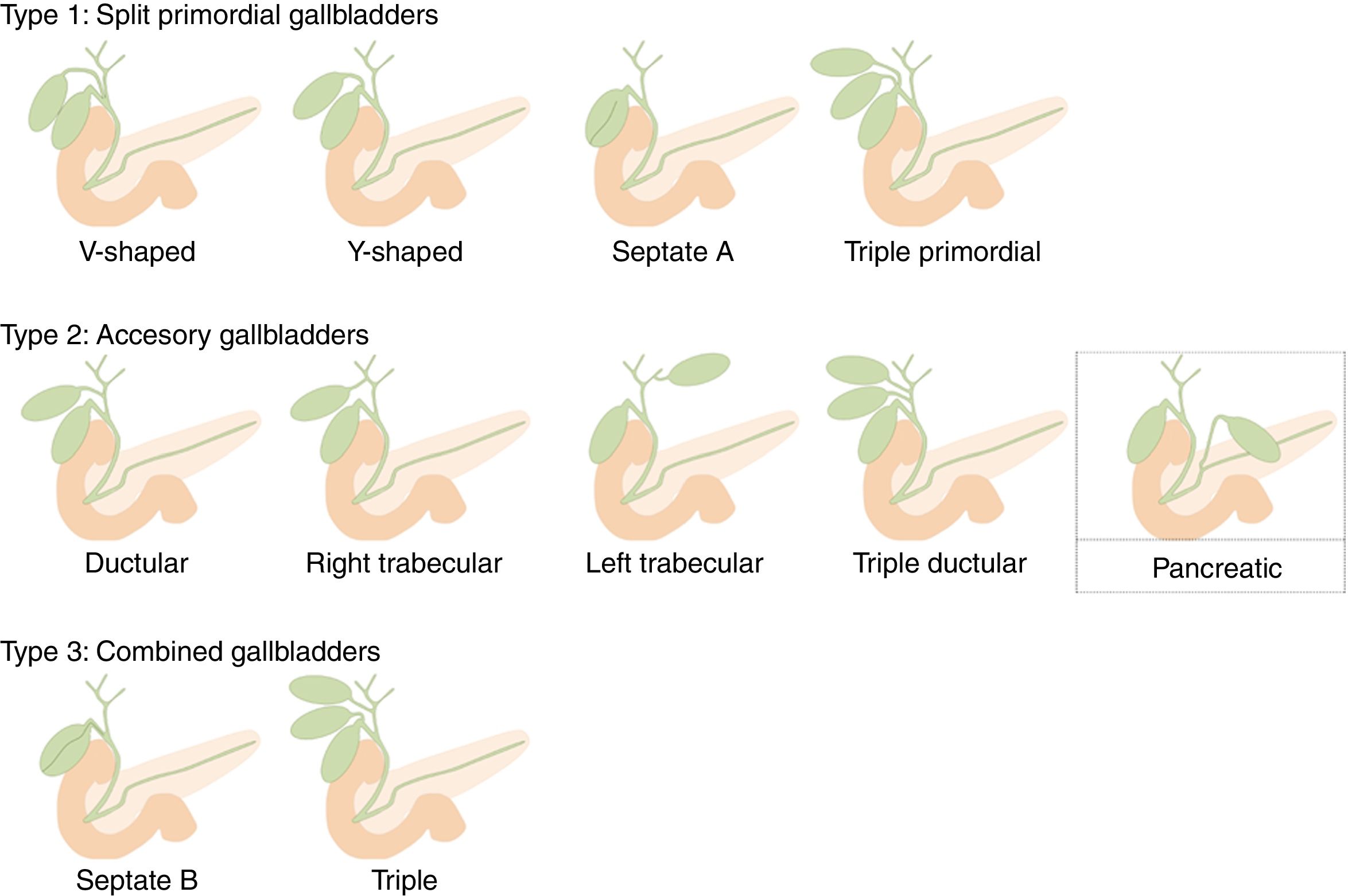
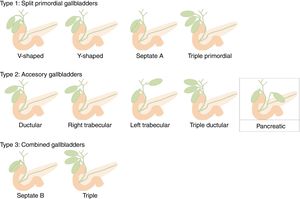
Gallbladder duplication is found in 0.0026% of autopsies. Duplication normally occurs due to extrahepatic bile duct evagination during the fifth and sixth weeks of gestation. These evaginations usually revert; however, sometimes they persist, giving rise to an accessory gallbladder.8 Harlaftis proposed an accessory gallbladder classification, which was subsequently modified by Causey et al. (Unified Classification of Multiple Gallbladders).9 It is divided into 2 groups according to embryogenesis. In type 1, the cystic primordium is divided during embryogenesis, and both gallbladders share a common cystic duct. Type 2 describes accessory gallbladders that can be ductular or trabecular, which means that they arise from a primordium separated from the bile duct and have separate cystic ducts. Type 3, proposed by Causey, includes combined forms of groups 1 and 2. As an accessory gallbladder, we propose that our case is a new variant that should be included in type 2. We have called it ‘pancreatic’ as it drains into the pancreatic duct and not the biliary tree (Fig. 4).
Accessory gallbladders can develop the same pathologies as primary gallbladders, including cholelithiasis, empyema, cholecystocolic fistula, torsion, papilloma and carcinoma.10 Surgery would be indicated in conjunction with the primary gallbladder, using the same current indications for cholecystectomy. When an accessory gallbladder is found during classical cholecystectomy, it must be removed to avoid possible complications and recurrence of biliary colic. The current trend in type 1 and 3 accessory gallbladders is laparoscopic cholecystectomy. In type 2, however, some authors recommend an open approach due to the increased risk of injury to the main bile duct and the right hepatic artery.11 Nevertheless, there are reports in the literature of laparoscopic cholecystectomies of type 2 accessory gallbladders with good evolution.10 In the case of accessory gallbladders with abnormal drainage to the pancreatic duct, we consider that laparoscopic surgery may be possible with meticulous dissection of the structures, identifying surgical references with an approach from the gallbladder fundus to the neck in order to isolate the gallbladder pedicle (‘dome down’ technique) and with intraoperative cholangiography. In the case described, we opted for an open procedure due to the coexistence of the teratoma in the liver.
Imaging tests play a decisive role in the preoperative diagnosis of accessory gallbladders. Ultrasound can raise the suspicion of gallbladder duplication when 2 cystic structures are observed in the gallbladder fossa, or when one of them is adjacent to the right hepatic lobe, with confirmed postprandial contraction of one or 2 of the structures. Even so, the diagnosis is difficult to establish, and the type of duplication cannot be determined.12 Computed tomography does not offer sufficient visualization of the biliary anatomy for diagnosis10 and may overlook the presence of accessory gallbladders. Endoscopic retrograde cholangiopancreatography is able to visualize communications with both the bile duct and the pancreatic duct. It has the disadvantage of being invasive and providing a high rate of false negatives.12 Intraoperative cholangiography accurately visualizes the biliary anatomy, thereby aiding in planning the surgical procedure, and reduces the probability of bile duct damage during cholecystectomy by 30%.10 However, its role in the diagnosis of accessory gallbladders is not clear10 and it would not be able to identify gallbladders communicated with the pancreas.
Magnetic resonance cholangiopancreatography is a widely used technique for the study of bile duct abnormalities as it is non-invasive and does not use ionizing radiation. The use of iv contrast with biliary excretion, such as gadolinium-EOB-DTPA, provides anatomical and functional information, since it is excreted to the biliary tree. If accessory gallbladders are suspected, the filling of the cystic structures with contrast confirms the diagnosis and allows them to be classified by observing the drainage duct. However, gallbladders that are not connected to the biliary system will not fill with contrast, as in our case. Since MRI is not invasive and is equivalent to ERCP to establish a diagnosis, it should be the first test to be carried out if an accessory gallbladder is suspected.12
Our patient presented a gallbladder in the usual location and a cystic structure adjacent to the left hepatic lobe communicating with the main pancreatic duct. Both had morphologies and histologies of normal gallbladders, so it is our understanding that the second should be called an ‘accessory gallbladder’. Therefore, we propose its inclusion in the Unified Classification of Multiple Gallbladders as type 2 (accessory gallbladders), of the left trabecular subtype with drainage to the pancreatic duct. Accessory gallbladders are a rare entity whose understanding is essential for correct diagnosis and surgical planning, as they can develop the same spectrum of disease as gallbladders.
Please cite this article as: Lozano Rodríguez Á, Caballero Díaz Y, Sánchez Ramos M, Larrea Olea J, Junquera Rionda P, Fornell Pérez R. Vesícula biliar accesoria con drenaje en el conducto pancreático principal: hallazgos por imagen, manejo quirúrgico y clasificación. Cir Esp. 2020;98:100–104.