Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) is frequently associated with coagulation impairment and perioperative blood transfusion. Our aim was to investigate the impact of each procedure step on hemostasis, as measured by rotational thromboelastometry™ (ROTEM), fibrinogen level and platelet count as a primary outcome, along with its relationship with transfusion needs.
MethodsA prospective longitudinal study was performed. Haemoglobin level, fibrinogen level, platelet count and ROTEM parameters: clotting time (CT), clot formation time (CFT), maximum clot firmness (MCF), α-angle (EXTEM, INTEM, FIBTEM) were measured before the procedure, at the end of cytoreductive surgery and after HIPEC. Appropriate statistical tests were used for comparison. A P<.05 was considered as significant.
ResultsForty-one women, with median age 54 (range 34–76) were recruited. Cytoreductive surgery was followed by a reduction of haemoglobin level from 11.4±1.5g/dl to 10.6±1.6g/dl, a reduction of serum fibrinogen level from 269±69mg/dl to 230±48mg/dl (P<.01) and MCF decline from 20±10 to 16±8mm (P<.01), in the FIBTEM test. HIPEC was followed by no hemostatic impairment. The number of packed red blood cells administered during patients stay kept a mild significant relationship with both fibrinogen level (ρ=−0.5, P=.002), and MCF EXTEM values (ρ=−0.43, P=.006), recorded after HIPEC.
ConclusionsThe mild observed hemostatic impairment appeared after cytoreductive surgery instead of HIPEC, involving surgical haemorrhage as the most likely responsible factor. Further studies are required to confirm a correlation between transfusion needs and postoperative hemostatic tests.
La cirugía citorreductora seguida de quimioterapia intraperitoneal hipertérmica (HIPEC) se asocia frecuentemente a alteraciones de la hemostasia y a elevados requerimientos transfusionales perioperatorios. El propósito de este estudio fue analizar los trastornos hemostáticos asociados a cada una de las fases de este procedimiento terapéutico mediante tromboelastometría rotacional (ROTEM), niveles de fibrinógeno y recuento plaquetario, así como su posible relación con las necesidades transfusionales.
MétodosSe efectuó un estudio prospectivo longitudinal. Se registraron niveles de hemoglobina, recuento plaquetario, niveles de fibrinógeno y parámetros tromboelastométricos: tiempo de coagulación (CT), tiempo de formación del coágulo (CFT), firmeza máxima del coágulo (MCF), y ángulo α (EXTEM, INTEM, FIBTEM). Las mencionadas determinaciones se realizaron: antes del inicio de la cirugía; al finalizar la cirugía citorreductora y al concluir la HIPEC. Se utilizaron los test estadísticos apropiados. Los valores de p<0,05 se consideraron estadísticamente significativos.
ResultadosSe incluyó en el estudio a 41 mujeres con una mediana de edad de 54 años (rango: 34-76). Tras la cirugía citorreductora se observó una caída de la tasa de hemoglobina desde 11,4±1,5 a 10,6±1,6g/dl; un descenso del fibrinógeno sérico desde 269±69 hasta 230±48mg/dl (p<0,01) y una reducción de MCF en FIBTEM desde 20±10 hasta 16±8mm (p<0,01). La HIPEC no se asoció a alteraciones hemostáticas. Se observó una moderada relación negativa entre el número de concentrados de hematíes administrados y los niveles de fibrinógeno (ρ=0,5; p=0,002) y los valores de MCF EXTEM (ρ=−0,43; p=0,006) registrados tras la HIPEC.
ConclusionesLas alteraciones hemostáticas observadas aparecen tras la cirugía citorreductora, probablemente a consecuencia de la hemorragia quirúrgica. Se requieren más estudios para confirmar una correlación entre las necesidades transfusionales y las pruebas de coagulación postoperatorias.
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has shown to improve the survival of selected patients with peritoneal carcinomatosis.1,2 This type of treatment is based on the surgical removal of all visible neoplasm, followed by an intraperitoneal perfusion of intraperitoneal chemotherapy, with the aim of increasing cytotoxic activity, reaching high intraperitoneal concentrations with limited systemic absorption.3–5
A total of 28% of the patients treated with this procedure present haemostatic complications,1 particularly excessive haemorrhage, which is the cause of 18% of repeated surgery. Up to 51% of patients4 may require a transfusion due to high intraoperative blood loss, caused by surgery and the appearance of perioperative coagulopathy, attributable to the loss of proteins in the peritoneal cavity, high fluid exchange and possibly the administration of hyperthermic chemotherapy.4
The haemostatic changes described are a decrease in antithrombin III levels and the platelet count, as well as alterations in common clotting tests. These tests only analyse the plasmatic phase of clotting, whereas viscoelastic tests, such as rotational thromboelastometry (ROTEM), show the physiological process of clotting much more accurately, maintaining a good correlation with perioperative haemorrhage, and facilitating suitable patient management.6–11
The purpose of this clinical study is to analyse the alterations in haemostasis which may appear in any one of the stages of the procedure, by determining their fibrinogen levels, platelet count, and ROTEM, as well as to investigate the potential relationship between haemostatic alterations and perioperative transfusion needs.
MethodsAn observational, prospective study was performed in a consecutive sample of patients with primary or secondary peritoneal carcinomatosis, who completed CRS followed by HIPEC in a single hospital centre.
The sample size calculation was conducted using basal serum fibrinogen concentration values, the variance of which was calculated from the first 20 cases. With a confidence level of 95% (a=0.05) and precision level of 7%, an expected ratio of 50% and an expected loss ratio of 10%, a sample of 41 patients was believed adequate for the study.
All the patients were over 18 years old and granted their consent in writing to be included anonymously in this study, which, due to its purely observational nature, did not require approval from the Ethics Committee of the hospital.
Prior to anaesthesia, all the patients received 0.2mg of morphic intrathecal chloride to manage their postoperative pain. The induction anaesthetic was done using propofol 2–2.5mg/kg, fentanyl 3–5μg/kg and cisatracurium 0.2mg/kg. The maintenance anaesthetic was composed of sevoflurane 0.5–1 CAM, remifentanil 0.25–0.3μg/kg/min and cisatracurium 1.2μg/kg/min. The invasive blood pressure, electrocardiogram, pulse oxygen, tele-respiratory CO2, oesophageal temperature and central venous pressure were monitored throughout the surgical procedure. Fluid therapy consisted of the administration of lactate Ringer at 15ml/kg/h. A transfusion of packed red blood cells was indicated with haemoglobin values <8g/dl. With the aim of preventing deep vein thrombosis in the lower limbs, a sequential compression device was used, so that patients did not receive heparin during the first 24 postoperative hours. Normothermia was maintained during surgery by warming fluids and a thermal blanket with a circulation of forced air at 39°C.
Once the CSR had been completed, during the HIPEC stage the abdominal cavity was irrigated with a solution of chemotherapy, consisting of cisplatin (75mg/m2) or paclitaxel (60mg/m2) for those patients with ovarian neoplasm, and mitomycin C (20mg/m2) for patients with colorectal cancer. The chosen chemotherapy treatment was diluted in saline, at a rate of 2l/m2 of body surface. The resulting solution was maintained at 41.5°C and underwent forced recirculation for 60–90min, using a Stockert S-III Double Headed Pump System (Stöckert Instrumente GmbH, Snia, Italy). Thirty minutes before the HIPEC stage, patients received methylprednisolone (1mg/kg), dexchlorpheniramine (2mg), ranitidine (50mg) and ondansetron (0.15mg/kg).
All the analytical observations conducted throughout the study were conducted using arterial blood samples taken before surgery (Stage 1); after completing the CSR (Stage 2), and 30minutes after completing HIPEC (Stage 3). The oesophageal temperature was recorded at the same time that each blood sample was taken.
Each sample was processed to obtain pH, calcaemia, haemoglobin levels, haematocrit, platelet count, fibrinogen levels and ROTEM. A ROTEM was done using a single device (ROTEM™ Gamma: Thromboelastometer unit TEM-International GmbH, Munich 81829, Germany), always by the same clinician, and within the first 10minutes of taking blood. Each sample underwent INTEM™, EXTEM™ and FIBTEM™ tests. All of these were programmed for a duration of 45minutes, and were conducted with the specific wells and reagents supplied by ROTEM™, following the instructions specified by the manufacturer. The following parameters were recorded for the INTEM and EXTEM tests: clotting time (CT), clot formation time (CFT), alfa angle (α) and maximum clot firmness (MCF). Only MCF was used for the FIBTEM test.
The statistical study was performed using the variance analysis for repeated measurements (ANOVA), applying the Bonferroni correction for post hoc comparisons. The presence of normal distribution was confirmed in advance with the Kolmogorov–Smirnov test, and the equality of variances of the differences between all possible pairs, with Mauchly's sphericity test. Spearman's coefficient of correlation was used as the measure of association between the number of packed red blood cells transfused throughout the stay in hospital, and the analytical data measured after the intervention (levels of plasmatic fibrinogen and MCF).
The statistical analysis was performed using the SPSS statistical software, version 22.0 (SPSS Inc., Chicago, IL, U.S.), considering values of P<.05 as significant. Quantitative variables were expressed as mean±SD, or rather as median and range, and categoric variables as the number of patients and percentage.
ResultsA total of 41 patients were included, Caucasian women, aged between 34 and 76 years (median: 54 years). A primary neoplasm was located in the ovaries in 31 patients (75.6%), colorectal in 7 patients (17.1%), and 3 patients (7.3%) presented peritoneal pseudomyxoma.
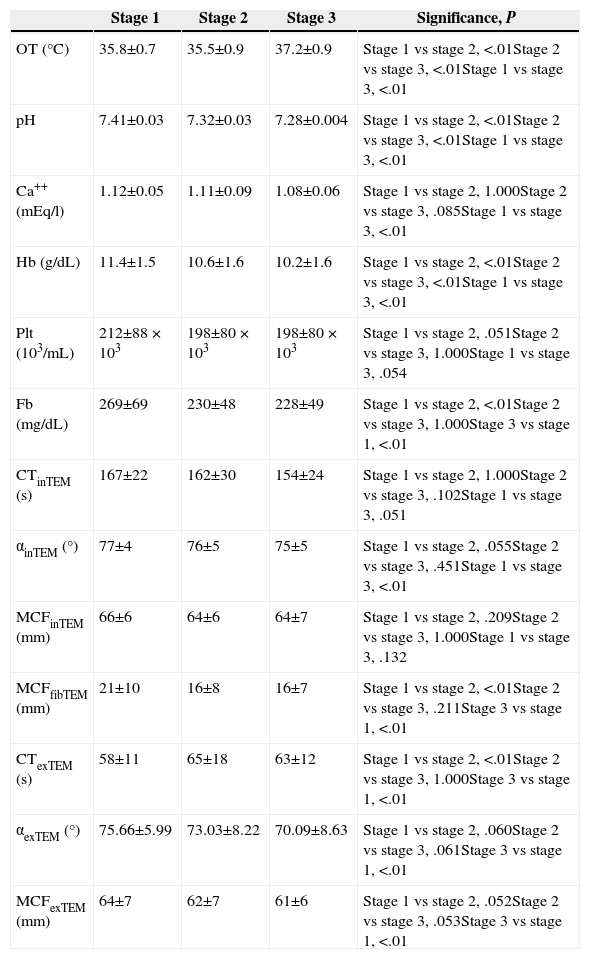
The peritoneal carcinomatosis index was 12.9±9, and the median duration of the surgical procedure was 336±86min (range: 180–550min). The preoperative creatinine clearance was 107.61±39ml/min, whereas preoperative prothrombin activity was 89%, as median, with a range of 62%–100%. The changes reflected in the parameters analysed are detailed in Table 1.
Summary of Results.
| Stage 1 | Stage 2 | Stage 3 | Significance, P | |
|---|---|---|---|---|
| OT (°C) | 35.8±0.7 | 35.5±0.9 | 37.2±0.9 | Stage 1 vs stage 2, <.01Stage 2 vs stage 3, <.01Stage 1 vs stage 3, <.01 |
| pH | 7.41±0.03 | 7.32±0.03 | 7.28±0.004 | Stage 1 vs stage 2, <.01Stage 2 vs stage 3, <.01Stage 1 vs stage 3, <.01 |
| Ca++ (mEq/l) | 1.12±0.05 | 1.11±0.09 | 1.08±0.06 | Stage 1 vs stage 2, 1.000Stage 2 vs stage 3, .085Stage 1 vs stage 3, <.01 |
| Hb (g/dL) | 11.4±1.5 | 10.6±1.6 | 10.2±1.6 | Stage 1 vs stage 2, <.01Stage 2 vs stage 3, <.01Stage 1 vs stage 3, <.01 |
| Plt (103/mL) | 212±88×103 | 198±80×103 | 198±80×103 | Stage 1 vs stage 2, .051Stage 2 vs stage 3, 1.000Stage 1 vs stage 3, .054 |
| Fb (mg/dL) | 269±69 | 230±48 | 228±49 | Stage 1 vs stage 2, <.01Stage 2 vs stage 3, 1.000Stage 3 vs stage 1, <.01 |
| CTinTEM (s) | 167±22 | 162±30 | 154±24 | Stage 1 vs stage 2, 1.000Stage 2 vs stage 3, .102Stage 1 vs stage 3, .051 |
| αinTEM (°) | 77±4 | 76±5 | 75±5 | Stage 1 vs stage 2, .055Stage 2 vs stage 3, .451Stage 1 vs stage 3, <.01 |
| MCFinTEM (mm) | 66±6 | 64±6 | 64±7 | Stage 1 vs stage 2, .209Stage 2 vs stage 3, 1.000Stage 1 vs stage 3, .132 |
| MCFfibTEM (mm) | 21±10 | 16±8 | 16±7 | Stage 1 vs stage 2, <.01Stage 2 vs stage 3, .211Stage 3 vs stage 1, <.01 |
| CTexTEM (s) | 58±11 | 65±18 | 63±12 | Stage 1 vs stage 2, <.01Stage 2 vs stage 3, 1.000Stage 3 vs stage 1, <.01 |
| αexTEM (°) | 75.66±5.99 | 73.03±8.22 | 70.09±8.63 | Stage 1 vs stage 2, .060Stage 2 vs stage 3, .061Stage 3 vs stage 1, <.01 |
| MCFexTEM (mm) | 64±7 | 62±7 | 61±6 | Stage 1 vs stage 2, .052Stage 2 vs stage 3, .053Stage 3 vs stage 1, <.01 |
α exTEM (°): alfa angle (degrees), measured in the EXTEM test; α inTEM (°): alfa angle (degrees), measured in the INTEM test; Ca++: calcaemia in arterial blood; CT exTEM (s): clotting time, in seconds, measured during the EXTEM test; CT inTEM (s): clotting time, in seconds, measured during the INTEM test; Fb (mg/dL): concentration of fibrinogen in miligrams per decilitre; Hb (g/dL): rate of haemoglobin in grams per decilitre; MCF exTEM (mm): maximum clot firmness (in mm), measured during the EXTEM test; MCF fibTEM (mm): maximum clot firmness (in mm), measured during the FIBTEM test; MCF inTEM (mm): maximum clot firmness (in mm), measured during the INTEM test; Plt (103/mL): platelet count in thousands per millilitre; OT: oesophageal temperature.
All the variables shown in the table follow a normal distribution, and were studied using a variance analysis for repeated measurements.
The CSR (Table 1: comparison Stage 1 vs Stage 2) was accompanied by a significant increase in oesophageal temperature (P<.01); significant decrease in arterial pH (P<.01); a drop in haemoglobin levels (P<.01); a reduction of serum fibrinogen concentration (P<.01); a decrease in MCF achieved in the FIBTEM test (P<.01) and a prolongation of CT in the EXTEM test (P<.01).
The HIPEC (Table 1: comparison Stage 2 vs Stage 3) was followed by increased oesophageal temperature (P<.01); decrease in arterial pH (P<.01), and a drop in haemoglobin levels from 10.6±1.6g/dl to 10.2±1.6g/dl (P<.01). The platelet count, fibrinogen and thromboelastometric parameters did not vary.
No packed red blood cells, platelets or other blood components were administered during the surgical stage. All patients stayed in the postoperative care unit for 24h, with a median hospital stay of 6.8±2.7 days. No patient died during the 30 days after the surgery.
During their hospital stay, 9 patients (21.9%) received packed red blood cells, with a median of 2 units (range: 1–3). A moderately negative correlation was noted between the number of packed red blood cells administered during hospitalisation and the levels of fibrinogen during Stage 3 (n=35; ρ=−0.5; P=.002). Likewise, a weak negative correlation was found between the number of packed red blood cells received and the values of MCF EXTEM gathered during Stage 3 (n=39; ρ=−0.43; P=.006). The haemoglobin levels at the time of discharge were 9.8±1.3g/dl.
DiscussionCSR with HIPEC has been associated with profuse perioperative haemorrhages, which has been attributed to the aggressive procedure and haemostasis impairment, possibly due to the high fluid exchange, loss of proteins in the peritoneal cavity and the impact of hyperthermic chemotherapy.4
Until now, haemostatic changes induced by this therapeutic procedure have been determined using clotting tests conducted at the laboratory, which were not developed for use during the perioperative period.9 Conversely, viscolastic tests, such as ROTEM, show the physiological clotting process more accurately, maintaining a good correlation with perioperative haemorrhage.6–11
When data obtained in the basal situation is compared to that obtained after completing CSR, it may be observed that, although they remain within normal limits, both fibrinogen levels and MCF FIBTEM values descend in a statistically significant manner. This finding appears to be a nonspecific consequence of surgical aggression, and matches prior studies, wherein the fibrinogen was the first factor of clotting to reach critical levels during progressive haemorrhage, as it occurs during surgical trauma,12 with MCF FIBTEM deterioration as a consequence of the decrease in the rate of plasmatic fibrinogen or a deterioration of its function.7,9
In this study, the deterioration of haemostasis appears to be milder than previously described,1,4 probably due to improved therapeutic procedures.13 This deterioration does not appear to be clinically relevant, even though results aim at a relation between transfusion needs and levels of fibrinogen and MCF EXTEM gathered during Stage 3, a finding in agreement with other studies.12
In this study, HIPEC is not followed by haemostatic changes, which is in contrast with prior studies that consider it to be one of the potential causes for haemostasis deterioration,4 although it is true that these studies have not attempted, as we have done in this work, to study the impact of CSR and HIPEC on haemostasis separately.
In this study, HIPEC was associated with a mild decrease in haemoglobin levels, which we believe had not been previously described. It cannot be explained by blood loss, since there was no visible bleeding during this stage of the procedure; nor is it likely due to hemodilution, since the rhythm of liquid infusion was kept constant throughout the whole procedure, and, furthermore, the duration of HIPEC is considerably lower than the CSR. It could be due to haemolysis, induced by intraoperative chemotherapy14 or by hyperthermia.
In patients treated with HIPEC, the oesophageal temperature was kept below 39°C, but the temperature reached and maintained inside the peritoneal cavity was in the range of the so-called “maximum human temperature,” meaning that the splanchnic vascular bed may have suffered the same thermal damage described in patients who experience a heat shock.15–17 Unfortunately, no peripheral blood smear was taken to look for schistocytes, nor any free haemoglobin quantified, data which, if positive, would have supported this hypothesis.
This research has several limitations: in the first place, only intraoperative changes were studied; in second place, it has not been possible to determine the influence of sex as a variable, and, in third place, the administration of steroids may influence haemostasis,18 which, in this study, appears to have been null, since haemostatic alterations appear at the end of cytoreduction, i.e. before the administration of steroids. It is worth asking whether, in spite of being mild, haemostatic alterations should be treated early, with the aim of reducing patient transfusion needs. Unfortunately, the sample size of this study does not allow us to identify a threshold of plasma, fibrinogen or MCF FIBTEM levels under which the administration of fibrinogen concentrate would achieve a reduction in transfusion needs, as has been shown in other clinical scenarios.19
In summary, the treatment of peritoneal carcinomatosis by CSR followed by HIPEC is accompanied by a reduction in serum fibrinogen and a deterioration of ROTEM parameters, mainly MCF FIBTEM. These alterations are mild and with limited clinical relevance, appear after CSR, and could be a consequence of the same. Further studies are required to confirm a correlation between transfusion needs and postoperative haemostatic tests in this subgroup of patients.
Conflict of InterestNone declared.
Please cite this article as: Falcón Araña L, Fuentes-García D, Roca Calvo MJ, Hernández-Palazón J, Gil Martínez J, Cascales Campos PA, et al. Alteraciones de la hemostasia durante la cirugía con quimioterapia intraperitoneal hipertérmica en pacientes con carcinomatosis peritoneal. Cir Esp. 2015;93:496–501.
Part of this paper was presented as an oral presentation at the ASA Annual Meeting 2011, in Chicago (USA) in October 2011.