Anorectal melanoma is an uncommon and aggressive disease. Because the patients often present with non-specific complaints, a high clinical suspicion is important to avoid a delayed diagnosis. Patients undergoing radical surgery have no significant survival difference compared to those undergoing wide local excision. Abdominoperineal resection should be reserved for selected patients in whom local excision is not technically possible or cannot obtain a clear margin. The indiscriminate use of groin dissection is not advisable in anorectal melanoma and should be used in selected cases. Systemic chemotherapy is generally a non-effective treatment and continues be studied. Radiation therapy can be used as hypofractionated radiation therapy combined with local excision or in a palliative setting. The oncological outcomes in anorectal melanoma are very poor. The aim of the present study was to review clinicopathology features and management of anorectal melanoma.
Melanoma anorrectal es un tumor infrecuente y muy agresivo. Su clínica es muy inespecífica, por lo que se requiere un alto índice de sospecha para evitar un retraso diagnóstico. La cirugía radical no ofrece ninguna mejora en la supervivencia y debe reservarse para aquellos pacientes en los que la escisión local no es factible. La linfadenectomía inguinal no está indicada de forma sistemática y debe valorarse de forma individualizada. La quimioterapia adyuvante no es efectiva. El papel de la radioterapia es controvertido. Puede utilizarse, bien como terapia hipofraccionada tras escisión local o bien como tratamiento paliativo. En cualquier caso, los resultados oncológicos son desalentadores. El objetivo de este artículo es realizar una revisión de la literatura existente sobre las características clinicopatológicas y el manejo del melanoma anorrectal.
First described 150 years ago by Moore,1 Anorectal melanoma (AM) is a rare tumor with very poor prognosis. Available scientific evidence on this type of neoplasm is heterogeneous and inconclusive. Furthermore, it is an entity that is difficult to diagnose clinically, due to the non-specific symptoms and because it shows as a non-pigmented lesion in one third of cases.2 Only a thorough examination, coupled with a high index of suspicion, will prevent delayed diagnosis.
All these factors mean that, unlike other anorectal neoplasms, AM lacks a fully defined therapeutic regimen. The biggest debate is based on the radical aspect of surgical resection (abdominoperineal resection [APr] compared to local excision [LE]) although, according to the most recent literature, survival is similar for both surgical options.3 In any case, oncological results obtained are not encouraging, with average survival not exceeding 15–20 months,4–6 and do not improve with any of the available adjuvant therapies (chemoradiation therapy or immunotherapy), although most patients with AM will die from distant metastases.
MethodologyA review was conducted of all existing literature using MEDLINE, Pubmed and Ovid databases until 2012. The following keywords were used: “Anorectal melanoma”, “anorectal neoplasm”, “abdominoperineal resection”, “local wide excision”, “anal canal”, “chemoradiotherapy”, and “radical surgery”. We have selected those items that, in the opinion of the authors, provided more conclusive scientific information. According to a very recent systematic review, until August 2012, a total of 2652 cases of AM had been published, most listed by specialized centers, with review periods not less than 40 years.7
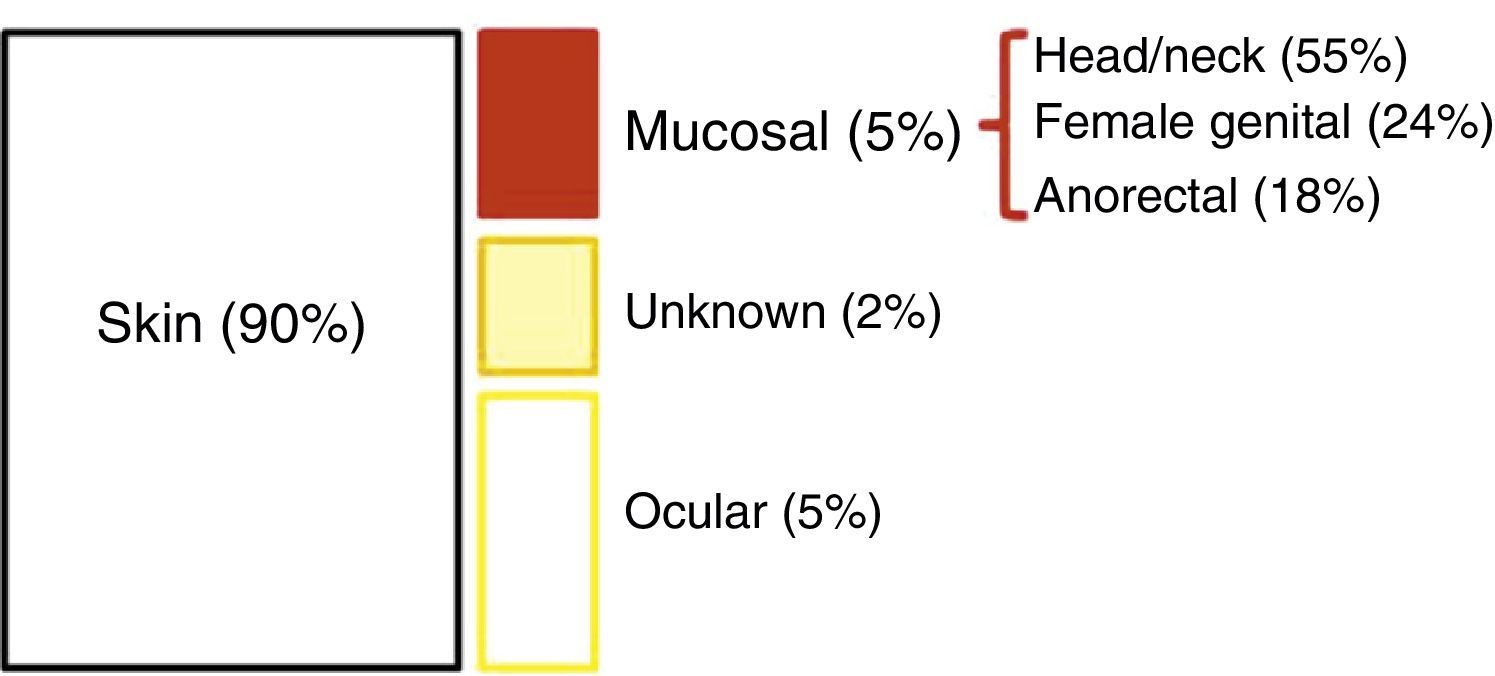
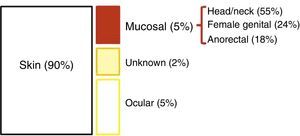
EpidemiologyEpidemiological data on AM is very heterogeneous. Its incidence (which has doubled in the last 20 years) in Western countries is about 1–2 cases per million inhabitants2,4; additionally, AM represents slightly less than 1% (0.1%–4.6%) of anorectal malignancies and will account for approximately 1%–2% of all melanomas.2,7–9 90% of melanomas reside in the skin; the remaining 10% is split between ocular melanoma (5%), melanoma of unknown origin (2%), and mucosal melanoma (3%). Among these, AM is the third most common site, after the head/neck and the female genital system4 (Fig. 1). However, among primary melanomas residing in the gastrointestinal tract, the anorectal location is the most common.10
AM is more common in women, with a 1.5:12,7,9 ratio, although some authors claim that this small increase results from perineal scans being more common among females.9 This disease usually affects elderly patients, with a peak incidence in the 8th decade of life (50% of patients), unlike cutaneous melanoma (CM), in which only 25% of patients are over 70 years old.2,7,9 However, there have been reports of AM in patients 11–19 years old,11 and an American institutional study, based on the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER), found an increased incidence of AM among young homosexual men (25–44 years old); therefore, it points to indirect evidence linking AM and HIV infection.12
Pathogenesis, Risk Factors and Histology. Similarities and Differences With Cutaneous MelanomaThe pathogenesis and risk factors for AM are poorly known, but some differences are sensed with respect to ocular and cutaneous melanomas, a fact that could have significant implications from a therapeutic point of view.7 In fact, epidemiological factors (CM is almost 20 times more common in Caucasians than in African Americans, while AM is only twice more common in whites) suggest that certain environmental risk components (e.g., ultraviolet radiation) clearly associated to CM are not involved in the development of mucosal melanomas in general, or anorectal in particular.7,9
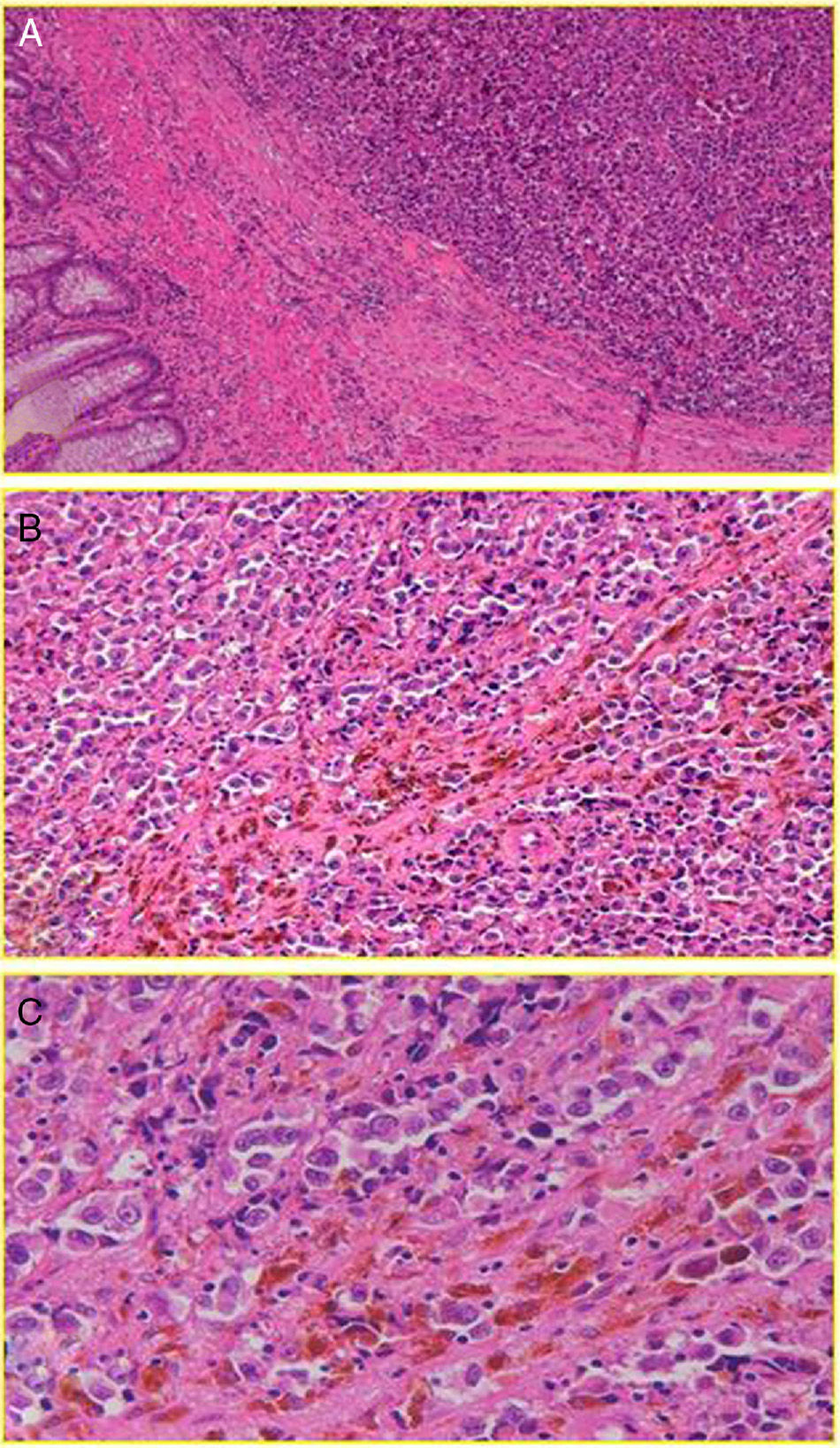
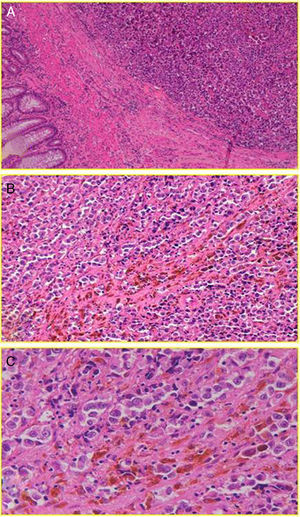
With regard to histology, we could claim that it does not seem identical to CM, although AM also has its origin in the malignant transformation of melanocytes; in this case, the anal canal. These are arranged in tumor niches that may be epithelioid (44%), mixed (31%) or spiculated (25%).13 Subsequently, these cells invade the squamous plane, expressing a number of melanoma immune-specific proteins, such as HMB-45, S-100 and vimentin2,8 (Fig. 2). However, in contrast to CM, a disproportionate number (up to 87% [10%–87%] based on most case series) of AM will be “amelanotic”.2,7 We do not know for sure whether or not this feature will have implications for prognosis (beyond an obvious decrease in the index of suspicion), although studies show comparable survival results for both types of lesions.14 Perhaps the most important thing is to emphasize the fact that not all pigmented lesions of the anal canal are malignant melanomas, and not all malignant melanomas are pigmented.
At the molecular level (particularly in the expression of the BRAF gene mutation) there are also differences between CM and AM. This suggests a different molecular pathogenesis for each of these entities, a fact that would have important diagnostic and therapeutic implications, especially for a possible gene therapy.15
Clinical SymptomsAM will present symptoms perfectly attributable to benign and much more common anorectal entities: rectal bleeding is the most common symptom, present in 53%–96% of patients, followed by the presence of a lump or mass, tenesmus, and more sporadically, itching, change in bowel habits or proctalgia.2,7,8,10 Associated symptoms, of banal appearance, will continue for an average of 3–8 months until the final diagnosis, and entails a misdiagnosis rate close to 55%.7 According to some studies, mistaking AM for hemorrhoidal disease has a statistically significant and negative impact on survival figures.6,7,16
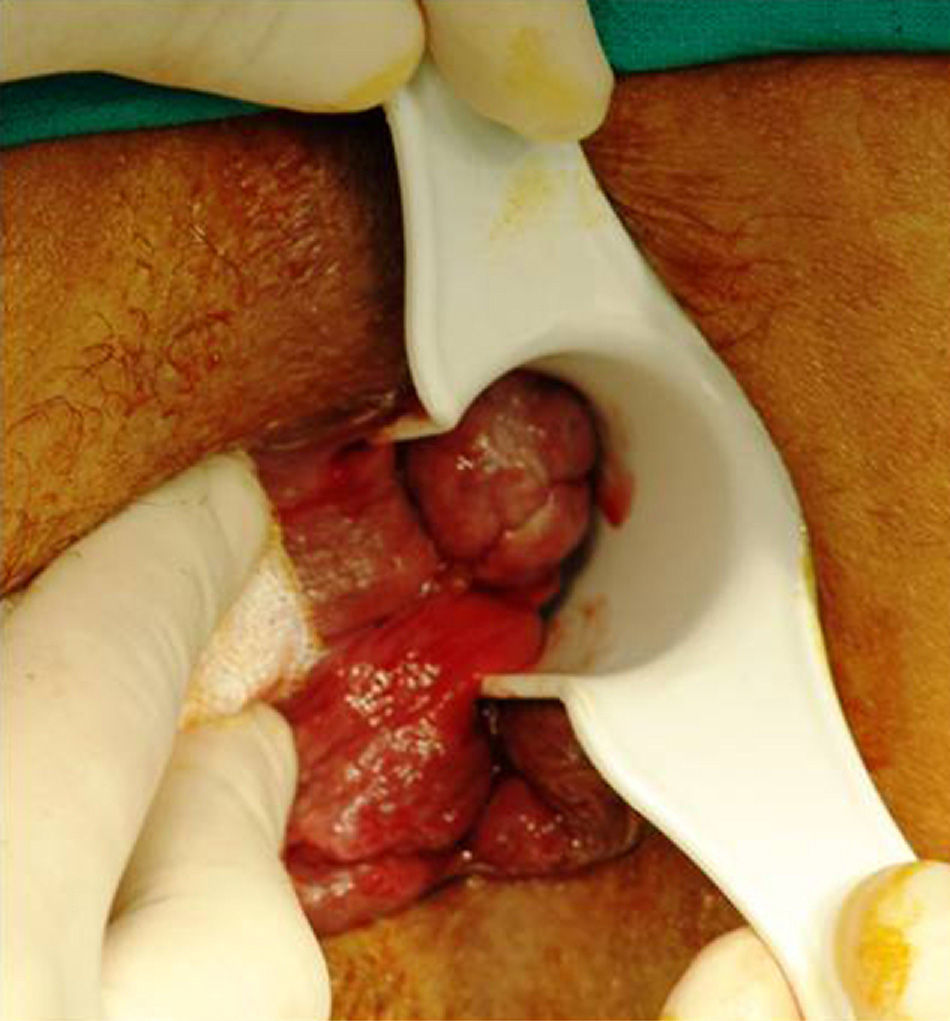
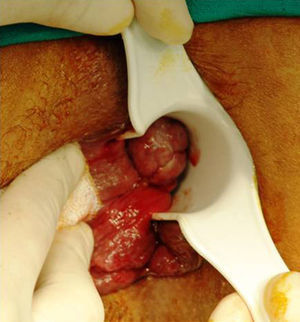
Anatomically, the majority of anorectal melanomas are located in the anal canal or in the dentate line. Only 2%–5% reside exclusively in the rectal mucosa.7 Usually, they are tumors (pigmented or otherwise) of 2.9–3.8cm in diameter6 with an ulcerated, flat or polypoid appearance (Fig. 3). A pigmented and polypoid lesion can be easily mistaken for a thrombosed hemorrhoid. Indeed, in an extensive review of the Memorial Sloan-Kettering Cancer Center (MSKCC), 8% of AM diagnoses were performed after pathologic examination of hemorrhoidectomy parts; therefore, it is recommended to systematically analyze all resected specimens and send them, identified topographically, to the pathologist.10,17
Diagnosis and Extension StudyAnuscopy and thorough anal examination (accompanied by a good index of suspicion) are critical in determining the size, location and characteristics (fixing, pigmentation, depth, etc.) of the lesion. Some authors advise against isolated biopsy, because it is a highly vascular lesion with high probability of false negatives, and even iatrogenic spread.18
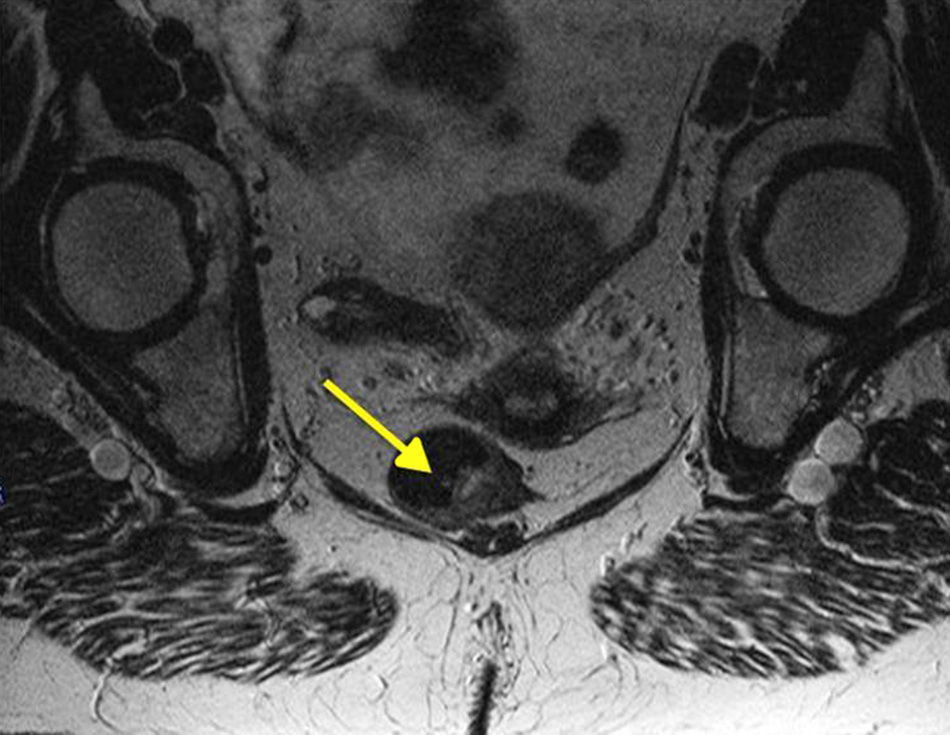
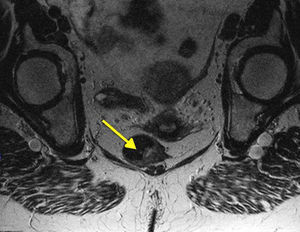
Moreover, nearly 20% of AM will have positive inguinal adenopathies and 7%–25% will begin with distant metastases (usually in the bone, lung, liver or brain). This highlights the importance of examination and analysis of the inguinal region as well as advanced imaging testing (pelvic MRI [Fig. 4], CT and endoanal ultrasound) to help us make decisions about treatment, although the clinical usefulness of these examinations, specifically for AM, has not been sufficiently documented.2,7,8 PET-scan has low sensitivity; therefore, most authors do not recommend its systematic implementation, saving it for doubtful lesions after CT.19
One controversial aspect is the thickness of the lesion which, according to some authors, is related to lymphatic spread, local recurrence after surgery, and oncological outcomes, with 33-month survival time figures for tumors <4mm thick, compared to 8 months for those >4mm.18,20 However, the literature is particularly heterogeneous in addressing this issue, using classifications referring to CM; some are obsolete (Clark's), others are more recent (Breslow's) or do not provide any data on lesion thickness which, according to a recent systematic review, occurs in more than half of the publications.10 Moreover, and given that unlike CM, most AM have a thickness of ≥4mm at diagnosis,6,13,17,20 the clinical and prognostic value of this parameter is questionable; therefore, the classification of the American Joint Committee on Cancer for CM does not apply to AM without a currently validated system for staging anorectal melanomas, although some authors simplify their classification into three classic stages (i) localized; (ii) spread to lymph nodes, and (iii) distant metastases).2,21 According to some studies, unlike with CM, for AM, the factor determining the probability of lymphatic spread or distant metastases is tumor invasion depth, and not lesion thickness17,21; therefore, a very recent systematic update proposes a new system that increases these stages from 3 to 4, including stage I if the tumor does not infiltrate the muscular layer, and II if, during the endosonographic analysis, it appears infiltrated7 (Table 1).
New Staging Proposed for Anorectal Melanoma.7
| Stage I | Tumor DOES NOT infiltrate muscular layer |
| Stage II | Tumor DOES infiltrate the muscular layer |
| Stage III | Locoregional lymphadenopathy |
| Stage IV | Distant metastasis |
Abdominoperineal resection, the classic AM surgical treatment, has never been validated prospectively. Indeed, the high morbidity associated with this procedure, and the “perception” that radical surgery failed to obtain significant survival-related advantages, led to questioning (currently rather well documented) the benefits of APR as the initial treatment option for AM. The first publication dates back to 1982. In it, Cooper et al.,22 after analyzing 227 patients, found similar survival rates for radical surgery and for LE. Since then, there have been multiple case series, general reviews4–6,8,21 and some systematic reviews3,10 that provide similar conclusions although, to date, there is no randomized controlled trial that demonstrates them unquestionably. A study published in 2010,23 performed by the American SEER and analyzing the results of 143 patients (51 APA compared to 92 LE), concludes that for AM surgical treatment, survival does not correlate with the radicality of surgery. Similarly, Nilsson et al.4 studied 251 patients (66 APR compared to 86 LE) diagnosed with AM and listed in the Swedish cancer registry, with a mean survival of 14 months, without statistical difference for both groups. The largest case series reached the same conclusions5,6 as well as some general reviews.2,8 Two systematic reviews corroborating these claims have also been published. Droesch et al., after only updating the case series that provided data on survival (14 studies in total, over the 30 years of the update), fail to show differences between radical local surgery in any of the tumor stages.3 A very recent one, published in 2012 by Kanaan et al., analyzed 21 publications, including nearly 700 patients, with a mean survival similar for APR and LE (21 and 20 months, respectively).10 However, we must note that there are series, such as those performed at MSKCC, that obtain a better survival for patients undergoing radical surgery, although this was not corroborated by an update of outcomes from the same institution.2,17
Another issue is local recurrence. It is an important issue, since the appearance of a local recurrence (and its symptoms: pain, rectal bleeding, incontinence, etc.) will have a major impact on the quality of life of these patients, a fact of some importance, especially when facing very short survival expectations.20 In many studies, no isolated local recurrence data was available, and in general, the literature provides more mixed results than those referring to the survival issue. There are many publications with significantly lower figures of local recurrence after APR,3,6,17,20,24 although other authors obtained rates that are independent of the type of surgery performed.4,21,25 These discrepancies are eliminated if we consider only those patients in whom an R0 resection was possible. In a Swedish institutional study, R0 was considered as obtaining a resection margin greater than or equal to 1cm, showing that, in these patients, the rates of local recurrence after APR or LE did not differ significantly. Obviously, APR obtains a greater number of R0 resections, but if it is possible to perform LE with sufficient margins, the local recurrence and survival rates are similar for both techniques.4
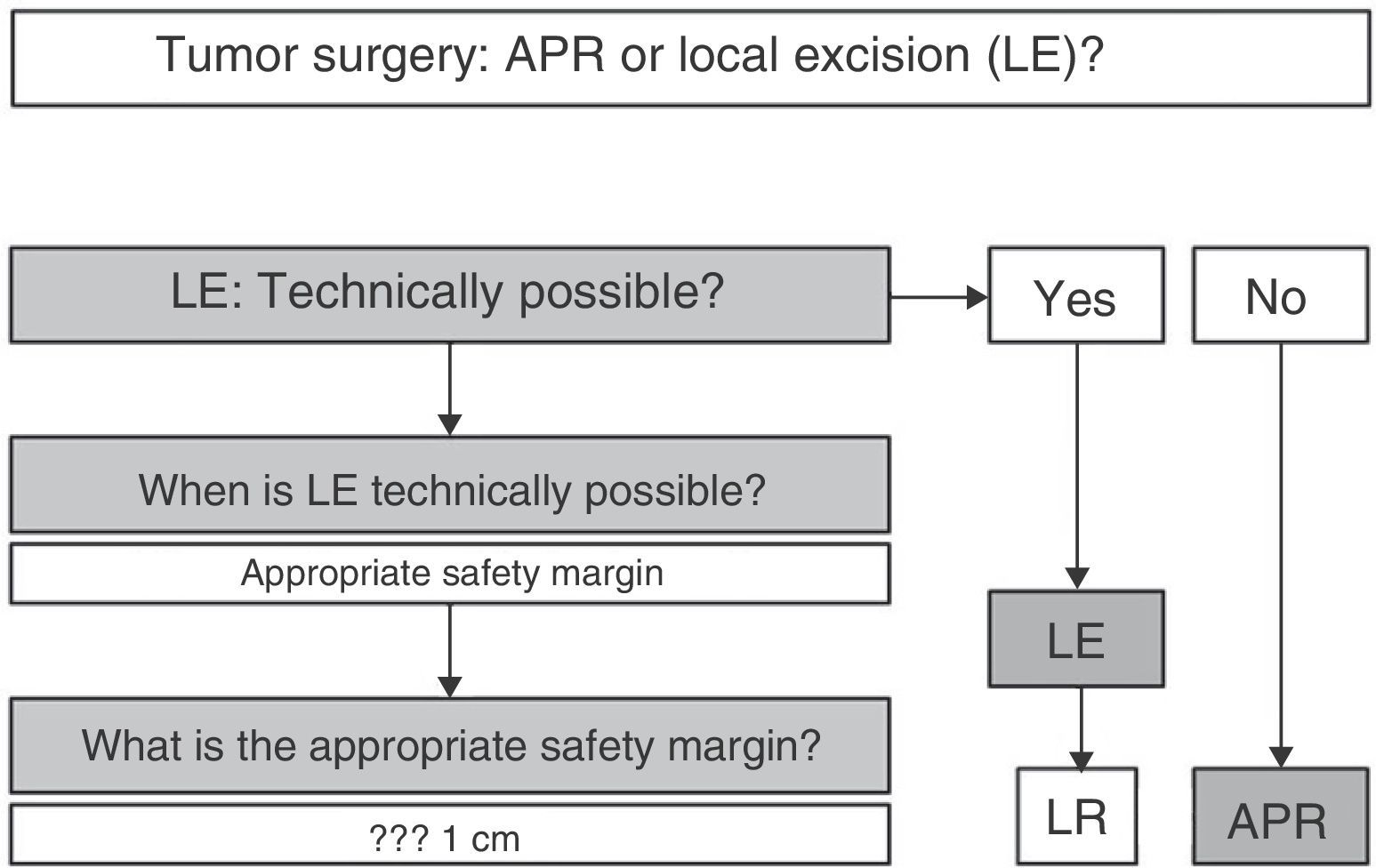
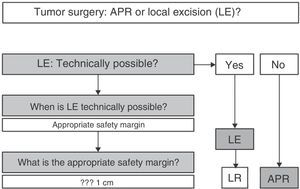
In summary (Fig. 5), and given that in AM, disease-free survival is going to be more related to the appearance of distant metastases than with local disease control, we can claim that, according to available scientific evidence, radical surgery in AM offers no survival improvement; therefore, APR must be reserved only for those patients for whom LE is not technically possible.2,3,7,8,10 LE is technically possible if it allows an R0 resection, i.e., an adequate margin of resection.4 In this sense, some authors provide guidelines where the margin depends on the melanoma invasion depth, advising LE and margin of 1cm in lesions smaller than 1mm thick, LE and margin of 2cm for lesions 1–4mm and APR for AM with a thickness greater than 4mm.20 However, the fact that most AM are >4mm thick at diagnosis (17 in 19 for the above-mentioned study), and that a high percentage of patients initially have lymph node and distant metastases, forces the decision to be individualized, but most authors consider an R0 resection if the resection margin is >10mm.4,21 In the case of local recurrence after LE, and in the absence of distant disease, APR may be chosen at onset.2,6
In any case, the decision must be individualized. It must be clear that an aggressive attitude does not improve survival; therefore, LE must be first choice, as long as the tumor characteristics (size, infiltration of the sphincter apparatus, etc.) make it technically feasible. Obtaining R0 resection is desirable, but not imperative.
A special situation would be the small number of patients (11%–18%) in whom the tumor is limited to the submucosa, without lymph node involvement according to the extension study (stage I, based on the proposed classification; Table 1). In these cases, according to a recent systematic review, radical surgery could be prescribed because these are the patients who, a priori, would have lower rates of spread and a more favorable prognosis.7
Lymph Nodes: The Role of LymphadenectomyIt is important to clarify that the “typical” AM lymphatic spread is unknown, with the possibility of spread to deep iliac or presacral mesorectal lymph nodes. There is also no evidence that performing an inguinal, mesorectal or pelvic lymphadenectomy entails any improvement in survival.13 A very recent retrospective case series at MSKC New York26 concludes that oncological outcomes after surgical resection do not correlate with the presence or absence of lymph node metastases.
Moreover, lymphadenectomies are not without morbidity. For inguinal lymphadenectomy in particular, the occurrence of complications such as lymphedema may impact the quality of life of patients. This leads to the fact that performing inguinal lymphadenectomy systematically is not prescribed for AM.2,9,26 However, selective indications are possible when palpable inguinal nodes exist without evidence of distant metastasis or involvement of other lymph node chains.9 One option is detection and dissection of the sentinel lymph node, however, this technique currently lacks sufficient scientific support, although it may be a promising advance in individualizing AM treatment.7,27
Adjuvant Therapy: Immunotherapy and ChemoradiotherapyMelanoma shows a greater immune susceptibility than tumors of other origins; therefore, it has been subjected to a wide array of immunotherapy-based trials, with some encouraging results, especially for advanced CM. However, for AM in particular, the use of immunotherapy (interferon, interleukins, vaccines, etc.), does not go beyond experimental therapy.2,9
AM does not respond to chemotherapy. The most common drug used, dacarbazine (or its oral administered equivalent, temozolomide) has shown responses reaching barely 20%, both for monotherapy and in combination with other drugs.9 The so-called biochemotherapy (combining a biological agent with conventional chemotherapy) has shown some usefulness in metastatic CM.27 In the series published by Kim et al., its application to AM, also with distant metastasis, showed some acceptable results: 11% complete response, 44% favorable response plus a 3-month increase in mean survival.28
The use of RT in the treatment of AM is very controversial. Although melanoma has historically been a radioresistant tumor, recent studies question this attribution. For AM in particular, an MD Anderson study published in October 201129 obtained a 17% local recurrence rate for the combination of local excision followed by hypofractionated RT (25–36Gy in 5–6 fractions), compared to 50% recurrence rate for LE as sole treatment, according to most of the case series. However, we must emphasize that, in this case series, improvements in local disease control did not correspond to an increase in survival because, as we have stated, the predominant pattern of recurrence for this disease is systemic recurrence. Moreover, the role of RT is indeed established as palliative therapy for 40%–50% of patients with unresectable, recurrent or disseminated disease, who develop accompanying symptoms, such as bone pain, spinal cord compression, tumor hemorrhage or CNS dysfunction.9
Prognostic FactorsThe data we have is quite heterogeneous. Factors such as size, duration of symptoms, thickness (on scales used for CM) or certain molecular markers have been implicated. There is nothing clear on the subject.5 Indeed, there is no unanimity that the presence of lymph node metastases has implications in the prognosis of AM,13 although many authors consider this fact a very negative indicator, especially if the lymph nodes are mesenteric,17 with an impact greater than that demonstrated for CM.4,29
However, some factors are clearly implicated. One is the invasion of the submucosa, which is not at all related to the “thickness” of the lesion and, as we know, allows access to lymphovascular channels and facilitates the spread of the tumor. Therefore, some authors believe that, as with adenocarcinoma30 and unlike CM, tumor invasion depth, and not the thickness of the lesion, is the factor determining the odds of lymphatic or distant spread, hence the survival of patients suffering from AM.7,21 Another factor closely related to the prognosis is perineural invasion of the primary tumor, linked in some studies to 100% recurrence, compared to 67% rates if this particular aspect was absent.9,26
Oncological ResultsAs we have seen, AM is a highly lethal disease. The numbers are grim: 80% recurrence after “curative” surgery, 12%–15% survival at 5 years, and 17–21 months median survival (30 months for stage I, and 22 months for stage II).2,3,10
ConclusionAM is a rare entity, and it has certain differences with CM. Its clinical symptoms are non-specific; therefore, a high index of suspicion is required to avoid diagnostic delay. Radical surgery offers no improvement in survival; therefore, APR must be reserved only for those patients in whom LE is not feasible. Inguinal lymphadenectomy is not routinely prescribed and must be assessed individually. Adjuvant therapy is ineffective. Using RT is controversial, and currently prescribed as hypofractionated therapy after LE, or as palliative treatment. In any case, oncological results are discouraging.
Conflict of InterestThere are no conflicts of interest by any of the authors.
Please cite this article as: Reina A, Errasti J, Espín E. Melanoma anorrectal. Revisión de conjunto. Cir Esp. 2014;92:510–516.