Inguinodynia or chronic postoperative inguinal pain is a growing problem between patients who undergo surgical repair of an inguinal hernia. The change in results measurement proposed by many authors towards Patient Reported Outcome Measurement has underlined the importance of chronic postoperative inguinal pain, because of the great limitations in everyday life and the huge socioeconomic impact that it causes. In this article a narrative review of the available literature is performed and the most relevant aspects about epidemiology, etiology prevention, diagnosis and treatment of chronic postoperative inguinal pain are discussed. A new management algorithm is also proposed. The variability in its incidence and clinical presentation makes diagnosis of chronic postoperative inguinal pain a very challenging issue. There is no standardized therapy and an adequate etiological diagnosis is key point for a successful treatment. There are many treatment options that have to be sequentially used and adjusted to each patient and their clinical features.
El dolor inguinal crónico posquirúrgico constituye una complicación de incidencia variable entre los pacientes intervenidos de hernia inguinal. La tendencia actual de medición de resultados en términos de calidad de vida (patient reported outcome measurement) ha puesto de manifiesto la importancia de esta complicación, debido a las limitaciones de actividad diaria e implicaciones socioeconómicas que genera. En este artículo se realiza una revisión narrativa de la literatura disponible en las plataformas PUBMED, EMBASE y Cochrane Library y se discuten los principales aspectos concernientes a la epidemiología, etiología, prevención, diagnóstico y tratamiento del dolor inguinal crónico posquirúrgico, proponiéndose un algoritmo para su manejo. Los pacientes que padecen dolor inguinal crónico posquirúrgico presentan un amplio espectro de manifestaciones clínicas y su diagnóstico supone un auténtico desafío. No existe un tratamiento estándar y el éxito del mismo radica en un adecuado diagnóstico etiológico para poner a disposición del paciente el amplio abanico de medidas terapéuticas de las que se dispone de forma individualizada.
Inguinal hernia repair is one of the most frequently performed surgical procedures, totaling more than 20 million procedures in the world.1 Thanks to the success of prosthetic techniques in reducing recurrence rates, there has been a change in perception among the scientific community regarding the measurement of results. Many authors advocate measuring outcomes in terms of incidence of inguinodynia or self-determined quality of life (Patient-Reported Outcome Measurement).2–4
Postoperative inguinodynia or chronic postoperative inguinal pain (CPIP) is defined as new-onset groin pain or pain with characteristics that are different from the preoperative pain, which persists more than three months after the initial surgery and which appears as a direct consequence of nerve injury or somatosensory system involvement after hernia repair.5,6 Patients with inguinodynia may experience significant limitations in their daily activities and quality of life.7 Therefore, this problem has important socioeconomic implications as it generates significant direct costs (derived from health care) and indirect costs (temporary/permanent disability, lawsuits, etc.).8
This article provides a narrative review of different aspects concerning the epidemiology, etiology, prevention, diagnosis and treatment of CPIP.
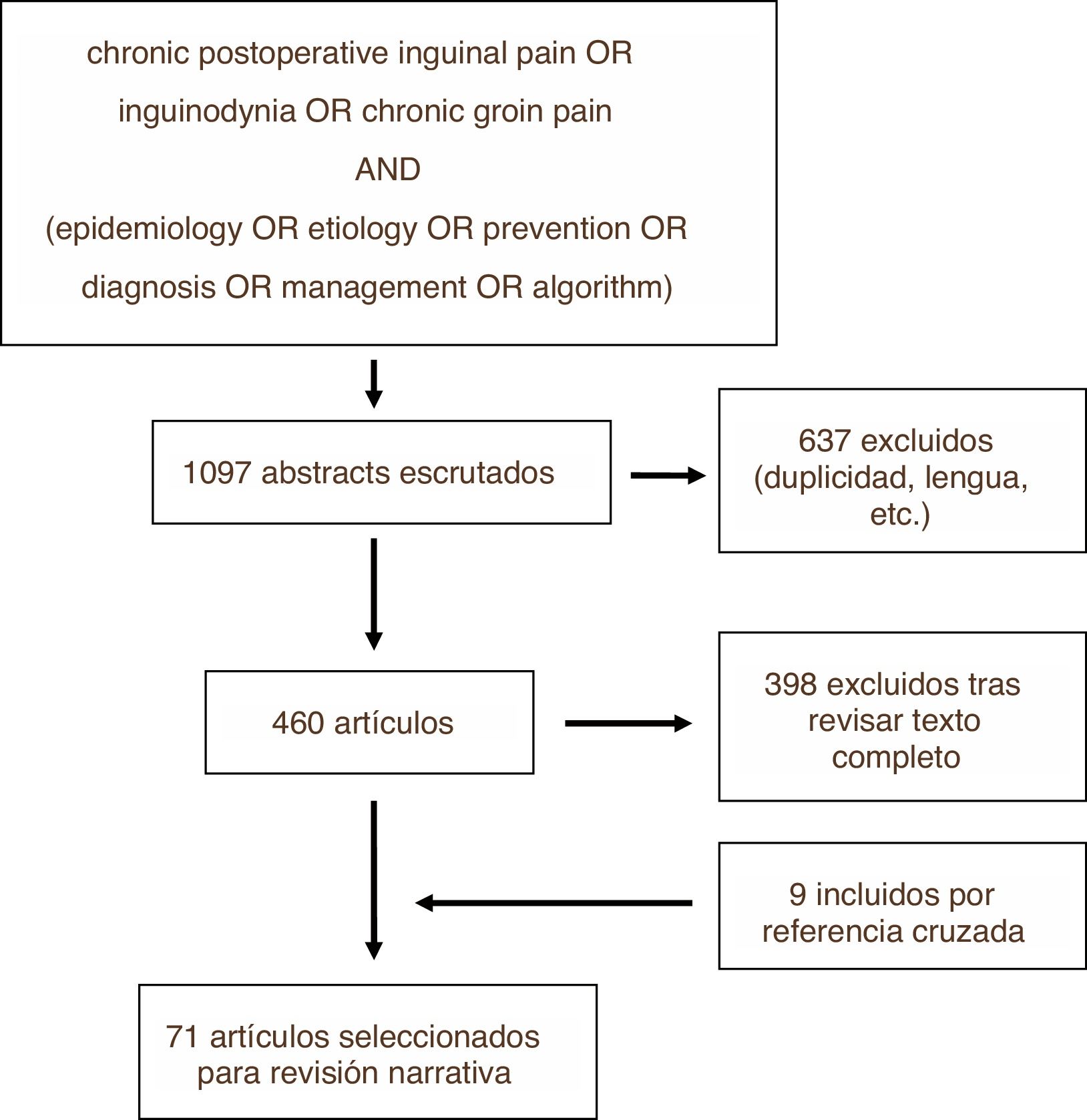
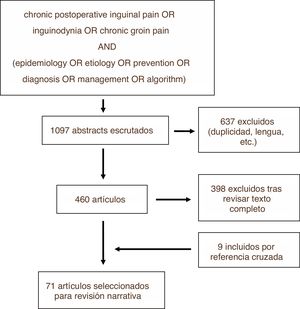
MethodsWe conducted a review of the literature on the PUBMED, EMBASE and Cochrane Library platforms using the search strategy shown in Fig. 1. The search was carried out in December 2018. Among the articles identified, we excluded those that did not meet the objectives of this review, as well as those written in a language other than English or Spanish, subsequently carrying out the narrative review based on the texts of the articles not excluded and prioritizing for this purpose those with greater methodological quality. This process was repeated with some of the cross-references from these studies, which were added to the narrative review if they were considered relevant. The review was carried out by the first two authors independently, and the third author resolved any discrepancies. Recommendations from clinical guidelines and the results obtained from randomized controlled trials were prioritized.
ResultsOut of the total of 1097 studies found with the search strategy described, in the end 71 were finally selected to become part of this review, either because of their methodological quality or because of the relevance of their results or statements. Fig. 1 illustrates the process for selecting articles for review (Fig. 2).
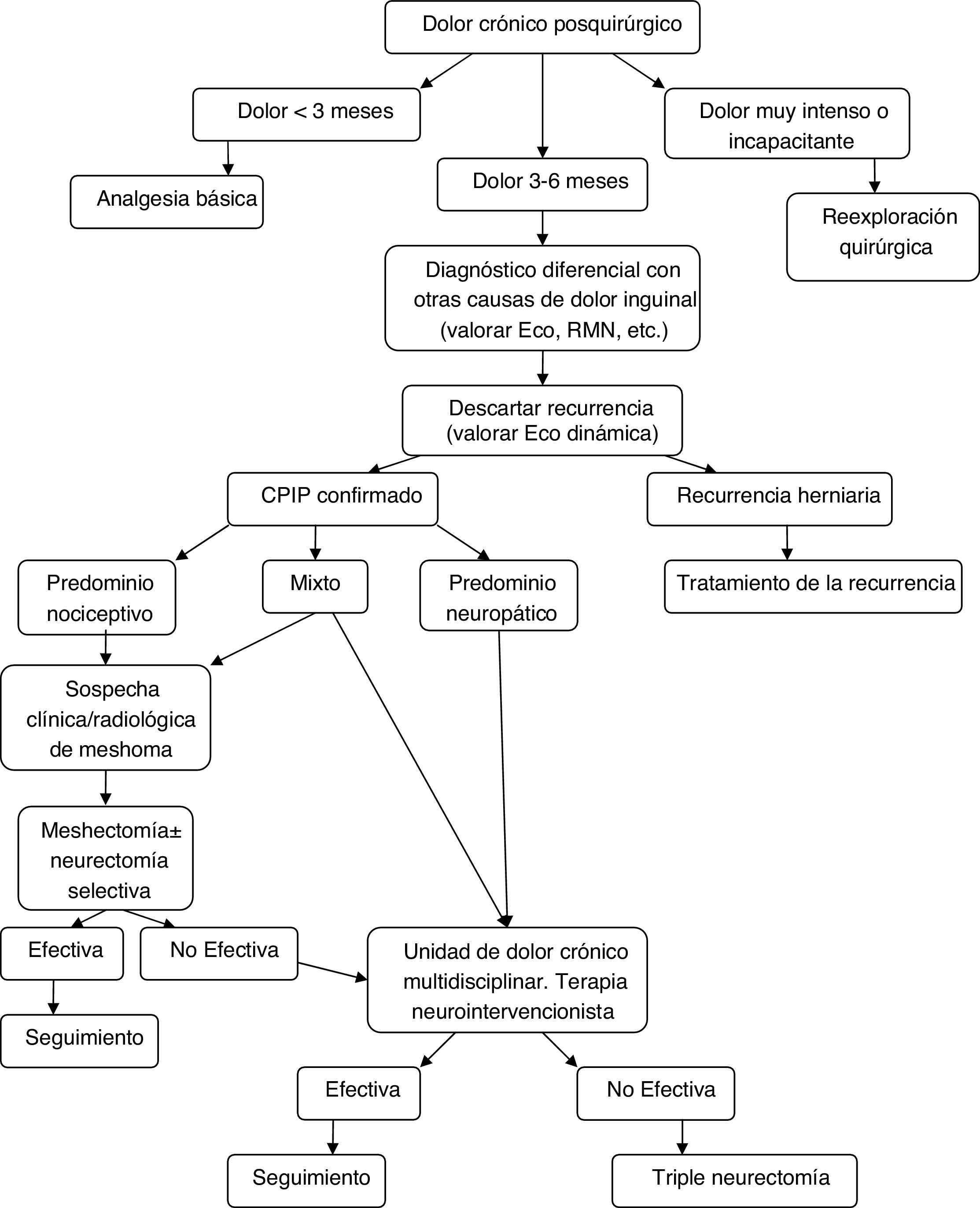
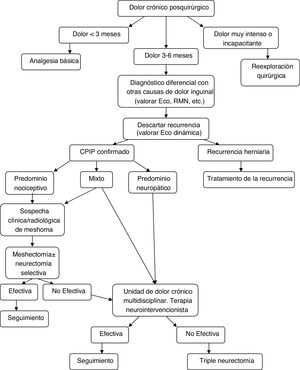
Diagnostic-therapeutic management algorithm for patients with CPIP, modified by Lange et al.15.
Very variable incidences of CPIP have been reported depending on the surgical technique studied, the definition applied in each study, and the methods of evaluation and follow-up used. These figures range from 0%-76%,5,7,9–11 and it is estimated that between 10% and 15.2% of patients who present this complication have moderate-severe pain or pain that affects their daily activities.12,13 The natural history of the pain is decreasing, and both the intensity and its impact on daily life tend to decrease over time.9
EtiopathogenesisIn order to understand the causes of CPIP, it is necessary to know that the clinical presentation of this complication can be highly variable, and two fundamental types of pain have been identified: neuropathic and nociceptive. In its etiopathogenesis, different components may intervene that can cause neuropathic, nociceptive pain or pain that combines characteristics of both types. Knowing how to recognize the potential etiology of pain is key to establishing appropriate treatment.
Neuropathic pain tends to predominate among patients with CPIP, a fact favored by the great anatomical variability that exists in the arrangement of the nerves of the inguinal region, mainly the ilioinguinal and iliohypogastric nerves and the genital branch of the genitofemoral nerve.14 This type of pain can be caused by direct nerve injury or perineural irritation, generally caused by compression, fibrosis, prosthetic material, sutures, or other fixation elements.15,16
Predominantly nociceptive CPIP is generally caused by a periosteal reaction in the pubic area, due to scar tissue or by mechanical pressure generated around what is known as a ‘meshoma’, which is a mass formed by a retracting hernia mesh and periprosthetic fibrosis.16–18 Furthermore, hernia recurrences can also present in the form of predominantly nociceptive pain.19,20
Other presentations of CPIP of visceral origin have been described in the form of orchialgia or dysejaculation, with a non-negligible incidence (approximately 10%).21,22 A proposed pathogenic mechanism is the erosion of the spermatic cord structures by the mesh, involving autonomic innervation of the vas deferens.22,23
Risk factorsSome systematic reviews of prospective studies and randomized trials have identified the endoscopic approach to inguinal hernia (transabdominal preperitoneal [TAPP]; totally extraperitoneal [TEP]) as a protective factor against the development of CPIP, compared to conventional repair techniques (prosthetic or not).7,24–26 In fact, the EndoHernia Society establishes a GRADE A recommendation for laparoscopic hernioplasty in order to reduce the incidence of CPIP.27
There is a technical aspect that has shown to behave as a protective factor with a high level of scientific evidence; fixation of the mesh with fibrin glues instead of other fixation methods in both open and laparoscopic surgery.28,29
Various risk factors have been proposed for the development of CPIP. Fränneby et al. identified the following risk factors: age below the median, a high level of preoperative pain and the appearance of immediate postoperative complications, such as seromas/hematomas or infections.30 These are, together with female gender, some of the risk factors for which the greatest consensus has been reached in the literature.10,11,31
Other potential influential factors that are not so unanimous are the performance of hernioplasty due to hernia recurrence,9,11 the type of mesh used32,33 or the appearance of pain or sensory dysfunction during the immediate postoperative period.34 A possible genetic influence has even been suggested.35
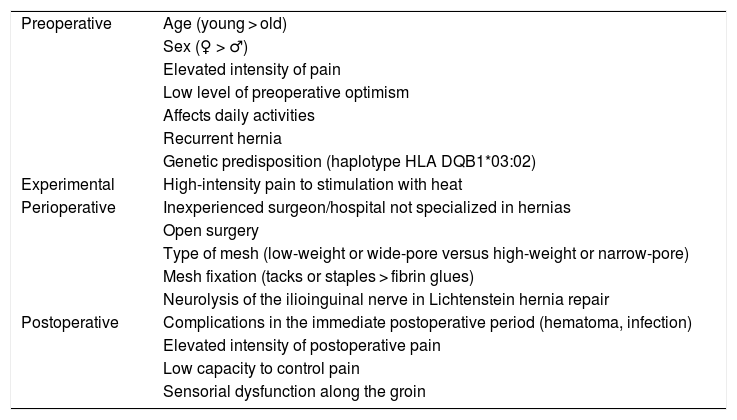
Table 1 summarizes the different risk factors identified in the literature for the development of CPIP.36
Risk factors for the development of postoperative chronic pain.
| Preoperative | Age (young > old) |
| Sex (♀ > ♂) | |
| Elevated intensity of pain | |
| Low level of preoperative optimism | |
| Affects daily activities | |
| Recurrent hernia | |
| Genetic predisposition (haplotype HLA DQB1*03:02) | |
| Experimental | High-intensity pain to stimulation with heat |
| Perioperative | Inexperienced surgeon/hospital not specialized in hernias |
| Open surgery | |
| Type of mesh (low-weight or wide-pore versus high-weight or narrow-pore) | |
| Mesh fixation (tacks or staples > fibrin glues) | |
| Neurolysis of the ilioinguinal nerve in Lichtenstein hernia repair | |
| Postoperative | Complications in the immediate postoperative period (hematoma, infection) |
| Elevated intensity of postoperative pain | |
| Low capacity to control pain | |
| Sensorial dysfunction along the groin |
Adapted from Bjurstrom et al.36.
To prevent this complication, some authors have proposed the ‘watchful waiting’ strategy for selected patients with inguinal hernia. This strategy has shown a low incidence of complications like incarceration or strangulation in patients with asymptomatic or minimally symptomatic hernias in the short to medium term, with no major differences in terms of pain or quality of life compared to surgically treated patients. However, in the long term, a large part of these patients opt for surgical treatment due to symptomatic progression.37,38 In addition, the morbidity and mortality associated with surgical treatment of incarcerated or strangulated inguinal hernia must be considered.39 The HerniaSurge group recognizes that observation can be safe and reasonable in correctly selected asymptomatic patients; they recommend assessing the time of intervention according to socio-occupational circumstances and the surgical risk of each patient.6
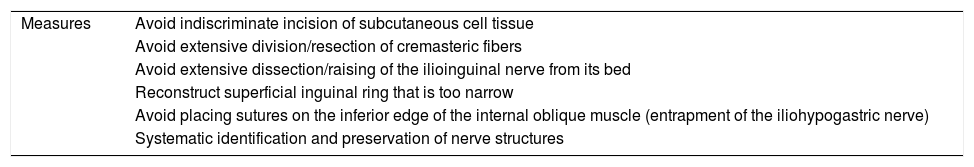
Several intraoperative measures have been proposed to prevent CPIP, especially regarding the management of the inguinal nerve structures. Table 2 shows the measures proposed by Amid, although not all of them are supported by consistent scientific evidence.16 Below, some of the proposed measures are listed with the level of evidence that supports them as well as the related recommendations made by the most recent clinical guidelines.
Intraoperative measures to prevent the appearance of CPIP proposed by Amid et al.
| Measures | Avoid indiscriminate incision of subcutaneous cell tissue |
| Avoid extensive division/resection of cremasteric fibers | |
| Avoid extensive dissection/raising of the ilioinguinal nerve from its bed | |
| Reconstruct superficial inguinal ring that is too narrow | |
| Avoid placing sutures on the inferior edge of the internal oblique muscle (entrapment of the iliohypogastric nerve) | |
| Systematic identification and preservation of nerve structures |
Source: Amid et al.16.
First of all, the need for systematic nerve identification during surgery has been raised and even whether their division or preservation is preferable once identified. The most relevant study in this regard is the Alfieri et al. prospective, multicenter cohort study that reported a significantly lower incidence of chronic pain in patients in whom the ilioinguinal, iliohypogastric and genital branch of the genitofemoral nerve were identified.40 This result was also corroborated by a subsequent systematic review.41 The HerniaSurge group has established a strong recommendation for the simple recognition of the nerve pathway, without complete dissection.6
As for prophylactic neurectomy, selective ilioinguinal nerve neurectomy has been the most widely studied. Three meta-analyses (two based on randomized clinical trials) address this issue with contradictory results and significant heterogeneity. Furthermore, some of them report a higher incidence of paresthesia-dysesthesia in the region, which makes it impossible to establish a recommendation in favor of this prophylactic maneuver.41–43 Apart from the ilioinguinal nerve, the available literature for prophylactic neurectomy of the iliohypogastric or the genital branch of the genitofemoral nerve does not allow any recommendation to be established.6
Another aspect studied is the so-called ‘pragmatic neurectomy’. This involves performing neurectomy in those cases in which the nerve has been injured or is at risk of entrapment by the mesh or any fixative element. Smeds et al. report a significantly lower incidence of chronic pain after 3 months among the patients in whom this maneuver was performed.44 Despite the fact that there are other cohort studies (without a control group) with contradictory conclusions,45 the latest guidelines on inguinal hernia management establish a strong recommendation in favor of pragmatic neurectomy.6
There are other known potential sources of predominantly nociceptive pain as a consequence of periosteal reaction. Several studies suggest that sutures that include the pubic periosteum for prosthetic fixation can trigger CPIP,6,18 and a strong recommendation is established to avoid this type of sutures.6
To prevent orchialgia, the latest guidelines strongly recommend minimizing manipulation and trauma to the spermatic cord, even though there is no solid scientific evidence to support it.6
Some perioperative analgesia strategies to avoid CPIP have also been evaluated. The preoperative administration of gabapentin or pregabalin is a promising measure, although it has hardly been studied.46,47
DiagnosisAny type of pain is a symptom that is highly subjective, both on the part of the patient (depending on their pain threshold, profession, daily activities, triggers, etc) as well as the surgeon when evaluating it. Therefore, when facing a patient with CPIP, we should not fall into the development of prejudices, transference, or countertransference.
First of all, we have to take into account the possibility that the pain is from a cause other than the surgery. The patient may have a pathology other than inguinal hernia that could be the cause of pain even before the operation. Only then will we be able to make an adequate differential diagnosis with other entities, for example musculoskeletal (enthesitis of the adductors, osteitis pubis, etc), genitourinary tract, peripheral nervous system (lumbar radiculopathy, obturator or pudendal nerve entrapment syndromes, etc), or other pathologies. Its mere suspicion may indicate the need for other complementary tests, such as ultrasound, MRI or even neurophysiological tests (electromyogram).19,48,49
Therefore, an exhaustive anamnesis and a thorough physical examination by an experienced surgeon are the keys to the differential diagnosis in a patient with suspected CPIP. It is these characteristics that will allow us, in the first place, to rule out other underlying pathologies and extract crucial data to guide the etiological diagnosis.15
Once another underlying pathology unrelated to the hernia or its treatment has been excluded, the next step is to rule out a recurrence. In addition to the clinical history and physical examination, complementary tests can be used, fundamentally dynamic ultrasound of the inguinal region, although some authors also defend the utility of herniography.15,50
From there, the characteristics of the pain will make it possible to differentiate between predominantly neuropathic or nociceptive pain, as well as the possible association with orchialgia or other accompanying symptoms. There is no clear distinction in many cases, and the etiological diagnosis can be further complicated in the presence of neuroplasticity phenomena, pairing of somatic and vegetative nerve fibers, and centralization of pain. To all this, we must add the influence of genetic, cognitive and socioeconomic circumstances.
Patients with neuropathic pain usually report features of burning or lancinating pain, radiating to the genital region or the medial side of the thigh, combined with symptoms such as hypo-/hyperesthesia, allodynia, or hyperalgesia. In contrast, nociceptive pain is better localized pain, usually continuous and described as a ‘tightness’ or ‘sensation of a foreign body’, especially frequent at the level of the pubic tubercle. Furthermore, it tends to be worse in certain positions (when crossing the legs) or relieved in others (in the supine position), especially in the presence of a ‘meshoma’.20,36 Likewise, it is important to evaluate the presence of symptoms that suggest some type of associated autonomic dysfunction, especially in the form of sexual dysfunction or dysejaculation, whose presence can also affect treatment.23
In cases of predominantly neuropathic pain, we can resort to diagnostic maneuvers such as nerve blocks or dermatome mapping, introduced by Álvarez et al. and subsequently validated as a useful diagnostic tool to distinguish between neuropathic and nociceptive pain, as well as to identify the affected nerve with greater probability.51,52 The main utility of dynamic ultrasound lies in its ability to rule out the presence of a clinically non-evident recurrence, although some publications highlight its ability to diagnose the presence of a ‘meshoma’.19,20 MRI could also be useful to orient the origin of pain.53
TreatmentDespite the advances made, the treatment of patients with CPIP continues to represent a challenge. The lack of consensus when defining whether a treatment is effective or not makes it difficult to interpret and compare the results of the different treatments. Therefore, results must be interpreted with caution. Currently, the recommendations continue to be based on case series and expert consensus statements.6,36
In the absence of universal treatment, each patient must be proposed an individualized therapeutic plan, adapted to the clinical characteristics and intraoperative findings, in the case of opting for surgical treatment. For this reason, both the clinical evaluation and the treatment must be conducted by an expert surgeon (the term ‘herniologist’ has been created to designate this figure).5,15,16,36 In any case, multimodal treatment and management strategies must be established by multidisciplinary chronic pain units.
Except in cases of pain of great intensity that is extremely disabling from the beginning, conservative treatment is the initial therapy, beginning with the administration of analgesia, topical therapy and non-pharmacological treatments, such as physiotherapy and cognitive-behavioral therapy.54
As for pharmacological treatment, there are few studies that evaluate its effectiveness in patients with CPIP specifically, but it seems reasonable to extrapolate its proven efficacy in patients with other types of neuropathic pain, such as postherpetic neuralgia or diabetic neuropathy.55 In the latest recommendations on the treatment of neuropathic pain, the International Association for the Study of Pain recommends tricyclic antidepressants (amitriptyline/clomipramine), serotonin-norepinephrine reuptake inhibitors (venlafaxine), and voltage-gated calcium channel blockers (gabapentin/pregabalin) as the first line of treatment.56 Meanwhile, topical capsaicin or lidocaine are second choices due to their reduced efficacy and potential adverse effects.57,58 Non-steroidal anti-inflammatory drugs (NSAIDs) or even corticosteroids are used for the treatment of nociceptive pain, with varying degrees of efficacy. However, their spectrum of adverse effects limits their prolonged use.36
Neurointerventional treatments are also considered part of conservative multimodal treatment and may be useful in selected patients. However, the scientific evidence supporting these methods comes mainly from small series.
Nerve blocks with local anesthetic of the ilioinguinal or iliohypogastric nerve are a measure already mentioned as a diagnostic maneuver, but they can also be used for therapeutic purposes. Genitofemoral nerve block can also be performed, although it is more complex.59 The help of ultrasound or the use of neurostimulators allows this maneuver to be performed with greater precision, reducing adverse effects and the quantity infiltrated. However, the studies evaluating its efficacy are contradictory.60,61 In any case, based on the results of a recent clinical trial, it appears that surgical treatment with selective neurectomy is more effective than nerve block.62
Neuroablation (radiofrequency, cryoablation) are another alternative, especially in cases where nerve blocks manage to relieve pain for a limited time.36 However, the scientific evidence supporting these techniques is limited, according to a recent systematic review on pulsed radiofrequency neuroablation.63
The latest neurointerventional alternative is peripheral or central neuromodulation. Although the mechanism by which it achieves its benefits is not known,64 all the published series present favorable results in terms of pain relief, quality of life and reduction of analgesic consumption.65,66 Despite its effectiveness, the complexity of the treatment, its cost, and its limited availability mean that potential candidates must undergo a strict selection process. In it, psychosocial factors (motivation, social and family support, ability to follow the recommendations, etc), as well as the response to a preliminary test,64 must be evaluated. All of this can act as a bias when interpreting the promising results.
The reasonable time to extend conservative treatment measures depending on the response to the different options is 3–6 months,15 although some authors propose extending it up to one year.5,67 Subsequently, a surgical treatment may be necessary, for which we have different alternatives. To maximize the success of any intervention, it is essential for the surgeon to have extensive knowledge of the inguinal and retroperitoneal anatomy.68
Neurectomy is the surgical treatment of choice for patients with predominantly neuropathic CPIP. Favorable results have been published for both selective69 and triple neurectomy, either by the open inguinal16 or retroperitoneal endoscopic70 approaches. To date, only the Chen et al. prospective study has evaluated the efficacy of triple neurectomy, reporting subjective improvement in pain relief in the 20 patients who underwent endoscopic triple retroperitoneal neurectomy.67
There are no studies that compare selective and triple neurectomy. Critics of the selective technique allude to the anatomical variability of the nerve disposition, the important network of connections in the area, and the difficulty to identify (both clinically and intraoperatively) which nerve is responsible for the symptoms as reasons to advocate for triple neurectomy.15,71 It is recommended that the nerves be cut proximal to the old surgical field and that the nerve stumps be ligated after division and be ‘buried’ in the surrounding muscle to prevent the formation of neuromas or their regeneration.5,36,71 Some guidelines continue to leave the choice between triple or selective neurectomy to the discretion of the surgeon,6 while others opt for the triple.5
Although there are no comparative studies between endoscopic or open neurectomy, the retroperitoneal endoscopic approach combines the advantages of minimal invasiveness with a more proximal division of the nerve, which is more effective, especially in cases of CPIP after TEP or TAPP or a preperitoneal open approach.15,67,68 It is also possible to extend the neurectomy to the common trunk of the genitofemoral nerve in its retroperitoneal pathway over the anterior side of the psoas muscle using an open approach.71
Another surgical treatment option is the removal of the prosthetic material (mesh explant or ‘meshectomy’), which has been shown to be safe and effective in the long term for patients with nociceptive pain in the presence of a ‘meshoma’. It can be combined with a triple or selective neurectomy in patients who present mixed pain characteristics or in those cases with intraoperative evidence of nerve entrapment or injury.72,73 Selective neurectomy would also be associated if the position of the nerve interferes with the mesh removal. Some authors defend the usefulness of the explant alone, without associated neurectomy.20 Recently, cases of laparoscopic meshectomy have been reported for patients with CPIP after laparoscopic hernioplasty with good results.74 Mesh removal entails several risks. Apart from those inherent to surgical re-exploration of the groin (bleeding, nerve injury, testicular necrosis, etc) there is the potential risk of hernia recurrence. To minimize this, in most series some type of additional hernioplasty is used in association. For patients with CPIP after Lichtenstein hernioplasty or similar undergoing mesh removal via the anterior approach, recurrence rates of 0% have been obtained by associating an endoscopic hernioplasty, although the series were small.75,76
Another factor to contemplate when choosing the best surgical alternative is the presence of orchialgia or dysejaculation. Triple neurectomy does not appear to be sufficiently effective for these patients. As there is a greater etiological involvement of the prosthetic material and the fibrous reaction that it generates on the vas deferens and its innervation,23 mesh removal seems to be a better alternative. In refractory cases, resection of the vas deferens or orchiectomy may be necessary.15,23
With the information above and the appearance of studies after the latest therapeutic algorithm by Lange et al. that support it,20,73,77 we the authors believe that a more prominent role should be given to mesh explants (alone or associated with neurectomy) within the surgical treatment of CPIP, always based on the characteristics of pain and intraoperative findings. Fig. 2 shows a new diagnostic-therapeutic algorithm, adding these modifications to the algorithm proposed by Lange et al.15
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank Dr Gonzalo Gómez Guerra for his mentoring and transmission of knowledge in the field of abdominal wall surgery.
Please cite this article as: Medina Velázquez R, Marchena Gómez J, Luque García MJ. Dolor inguinal crónico posquirúrgico. Una revisión narrativa. Cir Esp. 2021;99:80–88.