Industry 4.0 offers new development opportunities for surgeons. Computer-aided design and 3 D printing allow for the creation of prototypes and functional end products. Until now, it was difficult for new devices to get to the manufacturing phase. Nowadays, the main limitations are our creativity, available spaces to test our creations and obtaining financing.
La industria 4.0 ofrece nuevas oportunidades de desarrollo a los cirujanos. El diseño asistido por ordenador y la impresión 3D permiten materializar muchas ideas y conceptos, facilitando la accesibilidad al diseño y la creación de productos, bien como prototipos, bien como productos finales funcionales. Hasta ahora era difícil llegar a la fabricación de nuevos dispositivos. En estos momentos la principal limitación será nuestra creatividad, disponer de espacios que permitan poner a prueba nuestras creaciones y lograr financiación.
In the Paleolithic era, Homo antecessor in Atapuerca used tools to perform activities and overcome the limitations of human hands. Tools made of sharpened stones, animal bones, fish bones, etc. provided the ability to dissect, cut and sew,1,2 which are the same manual tasks still performed by most surgeons today. The Egyptians and Romans contributed to the development of metal surgical tools, and from the Middle Ages until the 19th century few changes came about. In reality, in General and Digestive Surgery the greatest advances were made at the beginning of the 20th century with the incorporation of mechanical suture devices, later the incorporation of inert materials like synthetic mesh, and then at the end of the century with the use of tools for minimally invasive approaches, and subsequently the incorporation of robotic systems as these tools have advanced. The development of light sources, optical systems, platforms for different approaches and robotic systems, as well as advanced dissection-hemostasis equipment, enable us to conduct safe procedures with very few, small and sometimes non-existent incisions.
The recent emergence of new technologies associated with additive manufacturing or 3D printing systems has been a very important change. Initially, these systems are useful for improving surgical planning by creating anatomical models that reproduce the organ lesion (tumor or malformation) and its relationship with proximal viscera. This has been proven to reduce surgical time and surgical complications. 3D printing can also facilitate the manufacture of guides and even personalized prostheses, which is of great interest and already in full development in specialties such as Orthopedics, Maxillofacial Surgery and Neurosurgery.3–6 Of special interests to surgeons is how these technologies are able to contribute to the design and development of instruments and devices that could facilitate our work.6–9
Nowadays, there is much talk about 4.0 industry or the 4th industrial revolution, which is based on at least 4 technologies: (1) Internet of things, (2) Cyberphysical systems, (3) Maker culture (do it yourself), and (4) Factory 4.0. Obviously the devices of which we speak touch on at least 2 of these technologies: first of all, the maker culture based on new technologies, solid modeling and additive manufacturing; and, we could also say that its expansion would be facilitated by what is known as Internet of things, as the Internet could be used to share STL files and watch videos of operations using these devices, so that from any part of the world a team of surgeons could fabricate the device, observe how to use it and replicate the surgery.
IdeasMost surgeons over the course of their career have seen that their work could improve by implementing changes to existing instruments or creating new devices in order to conduct new surgical techniques. Our creativity (an example of which is how we constantly incorporate innovative techniques) has frequently been limited by the inaccessibility of the design process and use of materials to create prototypes.
There are ideas that attempt to address the need of patients as well physicians or the hospital with which they are associated. Permanent observation and awareness allow us to detect problems and imagine solutions.
Proven methods can be used to generate new ideas: brainstorming, idea structuring (6-3-5 method), SCAMPER (Substitute, Combine, Adapt, Modify, Put to another use, Eliminate, Reverse), etc. However, what is considered most effective is continuous training so we become accustomed to developing solutions to problems we encounter in our everyday activities; we need to learn how to think like artists.10,11 In fact, we should be constantly alert and thinking about how to better resolve healthcare problems that are a concern for our patients.
In our case, when using current devices for conducting transanal endoscopic surgery procedures, we have found that maintaining the necessary pneumorectum is sometimes difficult, and that a system that could provide mechanical distension of the rectum would make it unnecessary to insufflate CO2 and perform the procedure with spinal anesthesia.
From freehand sketches, engineers developed virtual models using solid modeling software, the design was refined, and functional prototypes were developed for testing, initially using mixed manufacturing (with pieces obtained by additive manufacturing in acrylonitrile butadiene styrene and machined parts in Al). The following link shows a video that summarizes this first phase of our development experience (https://www.youtube.com/watch?v=w9uqUp9k1d4).
In a second phase, the design has evolved into a device in acrylonitrile butadiene styrene, obtained exclusively by 3D printing, which will allow us to create a disposable, functional prototype (individualized according to size) in order to carry out clinical trials.
Computer-Aided DesignAlthough freehand drawing is still the basis of the design of any device (at least in its initial stages, to communicate the concept of the idea) and a skill much appreciated by surgeons, for some years now computers have allowed us to improve preliminary sketches and obtain easily modifiable three-dimensional figures. Computer-assisted or aided design is embodied by a set of computer tools that are developed to provide support for both conceiving designs and analyzing products as well as the manufacture of three-dimensional objects.
Although this type of software requires specific knowledge acquired in Engineering, Design or Graphic Arts, or Superior Training Certificate programs, it is also true that they use an intuitive visual environment that makes them attractive to those with an interest in design and creation. Programs that are extensively used in our country include Inventor, Solidworks, Solidedge, Catia or 3Ds max.12–16 However, it is recommended to attend an introductory course, since these programs are complex and self-learning can be frustrating. Nonetheless, time and dedication are necessary for a surgeon to acquire sufficient knowledge and skill to adeptly manage these program, so we recommend working with professionals and experts in graphic design or engineering who have acquired these skills.
Images can be obtained like those in Fig. 1, which is part of the device designed by our group with Solidworks.
Additive Fabrication or 3D PrintingAdditive manufacturing is a new group of manufacturing techniques that began to be developed at the end of the 1980s. Through this technology, an object is created by defining a sequence of layers.
3D printing was invented at the end of the 1970s. As in many other technologies, these machines were initially large, expensive and had several limitations. Now they are more affordable, reliable and smaller. The first 3D printer appeared around the year 1984, based on stereolithography and invented by Charles W. Hull. Later, with selective laser sintering in 1987, a metal powder was solidified by laser to create the desired object. Shortly afterwards, Fused Deposition Modeling technology appeared. Scout Crump used nozzles or heads and plastics or resins as a method to create three-dimensional objects. It was then that the commercial possibilities of this type of design and its products began to be seen. Within a few years, MIT patented “Three Dimensional Printing” (3DP) using a method similar to a standard inkjet printer, but with the ability to print in the 3 coordinates of space.16
Thus, we are approaching the possibility of creating figures (organs, body parts, etc.) based on computed tomography scans, magnetic resonance images, etc., in order to create guides or prostheses and to develop prototypes for medical devices and surgical instruments, which until now were products of computer-aided design.17
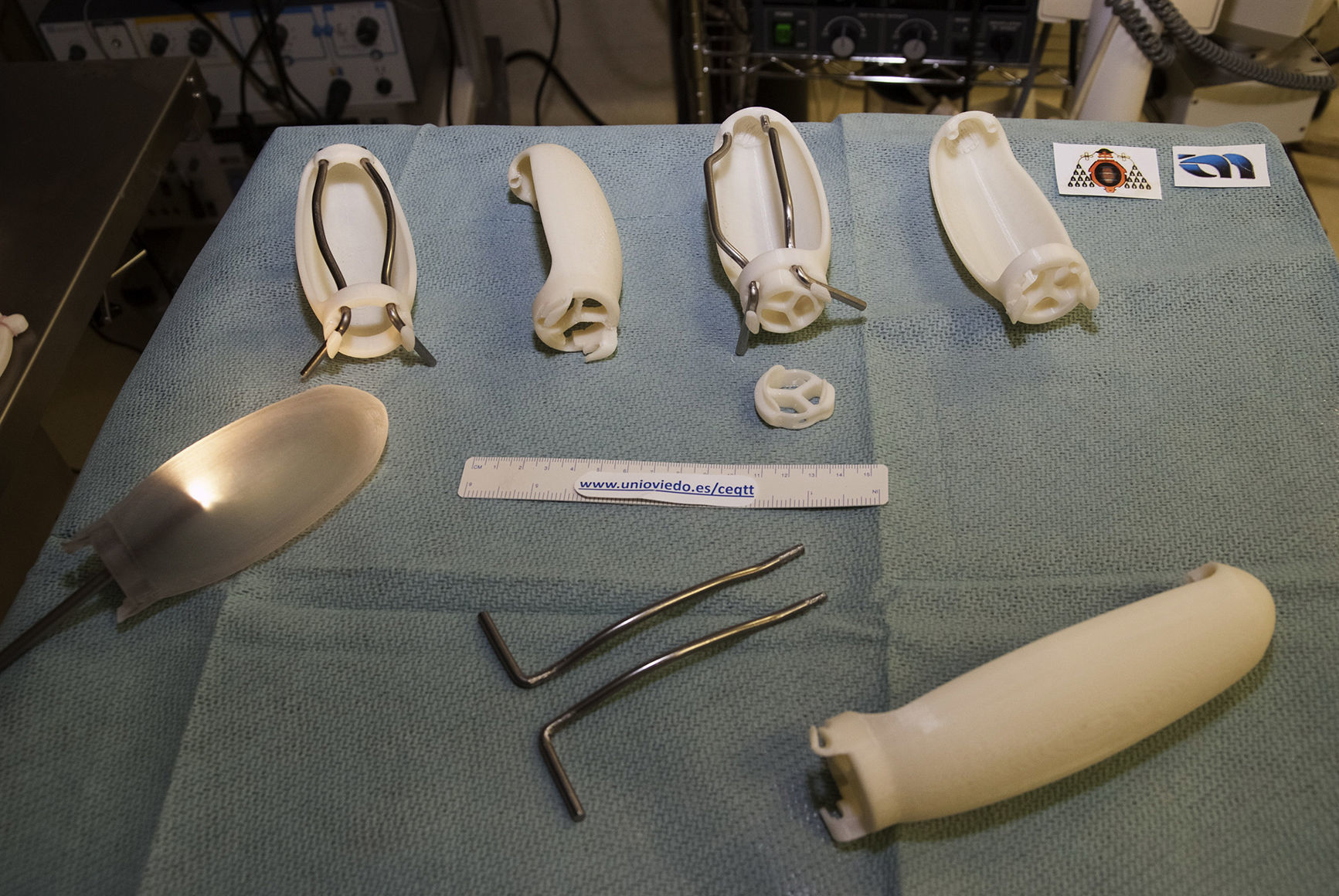
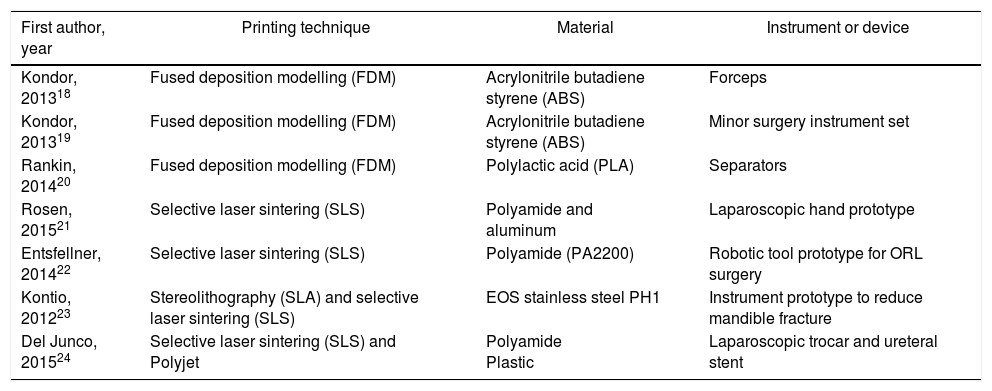
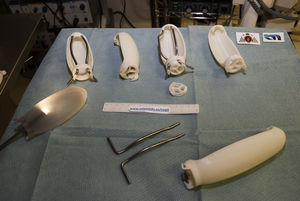
As in other disciplines, 3D printing has the advantages of being able to obtain short series, complex forms, customized devices and prototypes at low cost.16Fig. 2 shows some of the devices developed by our group using a 3D printer (HP Designjet 3D Printer), while Table 1 presents previous experiences in the surgical field.18–24
Design and Fabrication With 3D Printing of Surgical Devices and Instruments.
| First author, year | Printing technique | Material | Instrument or device |
|---|---|---|---|
| Kondor, 201318 | Fused deposition modelling (FDM) | Acrylonitrile butadiene styrene (ABS) | Forceps |
| Kondor, 201319 | Fused deposition modelling (FDM) | Acrylonitrile butadiene styrene (ABS) | Minor surgery instrument set |
| Rankin, 201420 | Fused deposition modelling (FDM) | Polylactic acid (PLA) | Separators |
| Rosen, 201521 | Selective laser sintering (SLS) | Polyamide and aluminum | Laparoscopic hand prototype |
| Entsfellner, 201422 | Selective laser sintering (SLS) | Polyamide (PA2200) | Robotic tool prototype for ORL surgery |
| Kontio, 201223 | Stereolithography (SLA) and selective laser sintering (SLS) | EOS stainless steel PH1 | Instrument prototype to reduce mandible fracture |
| Del Junco, 201524 | Selective laser sintering (SLS) and Polyjet | Polyamide Plastic | Laparoscopic trocar and ureteral stent |
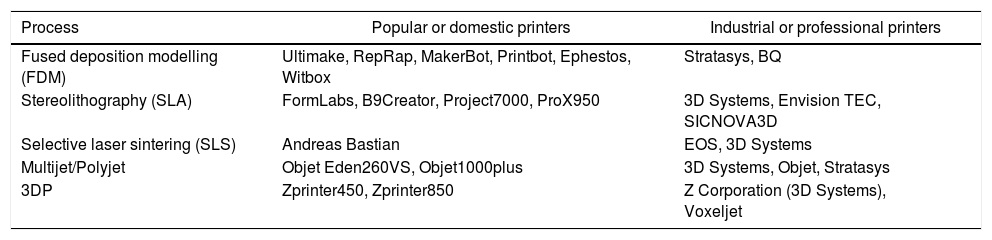
A multitude of 3D printers are currently available on the market. Table 2 shows the most representative models both for popular use and those that are considered professional or more advanced, as well as the printing technique they use.
Types of Printers Used Most According to 3D Printing Technique.
| Process | Popular or domestic printers | Industrial or professional printers |
|---|---|---|
| Fused deposition modelling (FDM) | Ultimake, RepRap, MakerBot, Printbot, Ephestos, Witbox | Stratasys, BQ |
| Stereolithography (SLA) | FormLabs, B9Creator, Project7000, ProX950 | 3D Systems, Envision TEC, SICNOVA3D |
| Selective laser sintering (SLS) | Andreas Bastian | EOS, 3D Systems |
| Multijet/Polyjet | Objet Eden260VS, Objet1000plus | 3D Systems, Objet, Stratasys |
| 3DP | Zprinter450, Zprinter850 | Z Corporation (3D Systems), Voxeljet |
Not long ago, the “Ballioll Collaboration” proposed the reference framework and the IDEAL Recommendations after a thorough analysis of the limitations of clinical trials in surgery.25 The fact that surgical innovations are performed manually by a professional and that there is the possibility of adverse effects, which are difficult to reverse (if not impossible), influence the evaluation of new techniques as well as the instruments and devices used. It is accepted that “the first time done in humans” can occur due to the need of an emergency or accidentally, but it is considered preferable to initiate practice with simulation systems (using synthetic organs or viscera, or from animals, etc.), with cadavers or research animals anesthetized in vivo under the care guidelines published by scientific societies. This allows for anticipating and avoiding injury and adverse results, as well as shortening surgery times, limiting expenses, etc. It is therefore essential to have spaces and equipment that enable us to test the prototypes developed.
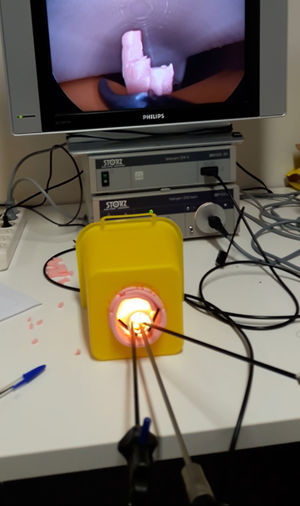
It is also essential to record the results (with increasing use of video recording and photographs) and create adequate databases accessible to all related professionals, preferably making them public in scientific fields of peers with proven critical capability.26Fig. 3 demonstrates how one of our devices is being tested in a mixed simulator. It is important to publish not only the successes but also failures in order to avoid others making the same mistakes.
The implementation of technical innovations has always concerned the clinical and scientific community, so it is subject to permanent analysis. In recent years, the most active and sensitive organizations have published recommendations and guidelines that seek to order and limit the adverse effects often associated with the introduction of new techniques and technologies.27,28
Until now, we surgeons had not considered introducing new devices or instruments into clinical practice, as companies were expected to do so. 3D printing can change this paradigm. There are so many possibilities with this technology that institutions like the European Community or the FDA have begun to consider the need for regulations in this regard.
First of all, it should be clear that all devices used and commercialized must have the EC mark, whose symbol must appear on labels, instructions or identification plates on the devices. In and of itself, this is an express declaration that the product complies with all essential requirements and any applicable conformity assessment procedures. In Spain, the regulation of medical products is essentially constituted by 3 Royal Decrees that stipulate the corresponding community directives that have been issued under the General Law of Health and the Law of Medicine, currently replaced by the Law of Guarantees and Rational Use of Medicines and Medical Devices. These Royal Decrees are:
- –
Royal Decree 1616/2009, from October 26, which regulates active implantable medical products, transposing Directive 90/385/CEE.
- –
Royal Decree 1591/2009, from October 16, which regulates medical products, transposing Directive 93/42/CEE.
- –
Royal Decree 1662/2000, from 29 September, about medical products for in vitro diagnosis, transposing Directive 98/79/CE.
Furthermore, in the development of new products (similar to new drugs), it is necessary to start with prototypes that will require experimentation and will have to be tested in viscera, animals or even humans, during the validation process of the device, before which approval from the governing Ethical Committees must be obtained.
Intellectual Property and PatentsUtility models, which do not exist in all countries, protect technical inventions of products with less inventive activity than those meeting patent requirements. They are characterized by their nation-wide novelty and relative industrial applicability. The protection is similar to patents, lasting 10 years and non-renewable. Utensils, instruments, tools, devices and their parts can be protected as utility models.
A patent is a temporary privilege of exclusive exploitation granted by the State for what is claimed in an application, if it meets the requirements required by law. New technical inventions that involve an inventive activity and are susceptible to industrial application are protected.29 For an invention to be considered patentable, 3 conditions must be met:
- –
Novel: This requirement is fulfilled when the invention is not included in the “state of the technique” (Article 6.1 Patent Law). Specifically, this means that there is no knowledge by written or oral description or history of use in our country or abroad. The novelty is related both to previous patents and to accessible references (books, journals, exhibitions, conferences, etc.).
- –
Inventive activity: This requires that the invention does not represent something obvious to an expert in the field, which could be obtained by uniting well-known technologies with a specific purpose.
- –
Industrial application: It is possible to manufacture the product and/or use it in some kind of industry. Diagnostic or therapeutic modalities applied to the human or animal body are not considered as such, including surgical techniques.
Patenting something presupposes the exclusive right to industrial and commercial exploitation of the object of the patent. It prevents any third party from manufacturing, offering, utilizing, commercializing or importing the invention without the owner's consent. The duration is 20 years, which is renewable with the payment of annual fees after its concession. In Spain, the concession organism is the Spanish Patent and Trademark Office (Oficina Española de Patentes y Marcas, OEPM), in Europe the EPO and at the international level the WIPO.
Funding and Business PlanObtaining funds to finance the development, production and commercialization of a product created by invention is difficult, especially when there are more financial losses and uncertainty at the beginning. There are several funding sources available:
- –
One's self: Investing in your own project is the best indicator of confidence and an unequivocal sign for other investors.
- –
Family and friends: People you know well, with whom you have a friendship, who can provide small sums of money to develop a second prototype, pay patent application costs, etc.
- –
Microinvesting or collective microfinancing: Group funding by investors who sympathize with the project and provide small donations. Generally, these involve social networks, online communities and micropayment technologies.
- –
Godfather or business investor and mentor: Willing to invest in risky projects with the ambition of obtaining beneficial returns or tax incentives. If this person has knowledge and experience in the sector, they also become a “validator”.
- –
Capital risk funds: These participate in businesses associated with dynamic sectors of the economy, with the aim to obtain above-average returns. Once the value of the company has grown sufficiently, they withdraw from the business, consolidating their return.
- –
Occasionally, there may be institutional support, such as from foundations, associations, or even prizes, contests, etc.
It is also possible to use marketing maneuvers in order to obtain orders before production, offering discounts for pre-paid orders, if necessary.
In any event, it is desirable to have a business plan detailing the steps to follow during a time and what is expected to be achieved as a business and/or at least a business model, which, with a more dynamic nature, would allow for changes to be made as different initial hypotheses become validated. Institutions like the Centros Europeos de Empresa e Innovación (CEEI) or their public and private equivalents can be especially useful.30,31
This last financing/business plan phase is where we are at this moment. It is here, in what the experts call the “valley of death”, where most projects die. Ours is still alive and fighting to find support.
As we have observed, the new 4.0 industry offers immense possibilities, while challenging not only our creativity, but also our capacity for innovation and entrepreneurship, which currently seem so necessary in our profession and socioeconomic context.
Conclusions and AcknowledgementsIn conclusion, we should transform necessities into ideas, and ideas into designs, which can easily materialize and even be functional with the use of 3D printing, although capable collaborators may be necessary. Likewise, we surgeons need to consider the possibility that we can be creative, not only in the development of techniques or procedures, but in the development of devices and instruments for innovation.
In our personal experience, our development of the distractor prototype has received funding from the Instituto Universitario de Tecnología de Asturias (IUTA, http://www.iuta.uniovi.es/web/). This led to the creation of a device patented by the University of Oviedo, requiring prior testing (patent number ES2503891-B2 and date of concession 06.IV.2015). At this time, we are actively searching for an industrial partner through the Office for the Transference of Research Results at the University of Oviedo (OTRI http://www.otri.uniovi.es/).
Last of all, we would like to thank Pablo Suárez Méndez for his effort and outstanding collaboration.
FundingThis study was partially financed by the Instituto Universitario de Tecnología Industrial de Asturias (IUTA). It contains scientific material and information presented at talks and oral communications at the 13th National Congress of Laparoscopic and Robotic Surgery–Award for Best Oral Communication (May 7–9, 2014, Chiclana de la Frontera-Cádiz), ASCRS Annual Scientific Meeting (May 30 to June 3, 2015, Boston), 14th National Congress of Laparoscopic and Robotic Surgery–Award for Best Oral Communication (May 11–14, 2016, Cáceres), at the 31st National Congress of the Spanish Association of Surgeons (November 7–10, 2016, Madrid) and the Meeting of the Minimally Invasive Surgery and Technological Innovation Division of the Spanish Association of Surgeons (May 11–13, 2017, Toledo).
Conflict of InterestAll the authors have contributed to the composition of the article and have approved the final version. None of the authors has conflicts of interests to declare.
Please cite this article as: Rodríguez García JI, Sierra Velasco JM, Villazón Suárez M, Cabrera Pereira A, Sosa V, Cortizo Rodríguez JL. Ingeniería de diseño en Cirugía. ¿Cómo diseñar, probar y comercializar dispositivos quirúrgicos fabricados con impresión 3D? Cir Esp. 2018;96:198–204.