Preoperative clinical staging is critical to select those patients whose disease is localized and may benefit from surgery with curative intent. Ideally, such staging should predict tumor invasion, lymphatic involvement and distant metastases. With the cTNM, we are able to select patients who could benefit from endoscopic resection, radical surgery or less radical treatment in patients with distant metastasis. The initial diagnosis of adenocarcinomas of the esophagogastric junction requires endoscopy with biopsies. For clinical staging, thoracoabdominal-pelvic CT scan, endoscopic ultrasound and PET or PET/CT are used. Other useful explorations are: barium swallow, endoscopic mucosal resection or endoscopic submucosal dissection (for assessment in initial stages) and staging laparoscopy. Once the resectability of the tumor has been established, the operability of the tumor should be assessed according to the patient's condition.
La estadificación clínica preoperatoria es crítica para seleccionar aquellos pacientes cuya enfermedad está localizada y se podrá beneficiar de una cirugía con intención curativa. Idealmente, dicha estadificación debería predecir la invasión tumoral, la afectación linfática y las metástasis a distancia. Con el cTNM podemos seleccionar aquellos pacientes a los que podremos ofrecer una resección endoscópica, una cirugía radical o evitarla en aquellos con metástasis a distancia. Para el diagnóstico inicial de los adenocarcinomas de la unión esofagogástrica se requiere una endoscopia con biopsias. Para la estadificación clínica: TC toracoabdominopélvico, ultrasonografía endoscópica y la PET o la PET-TC. Otras exploraciones de utilidad son: tránsito baritado, resección endoscópica de la mucosa o disección endoscópica de la submucosa (para valoración de estadios iniciales) y la laparoscopia de estadificación. Una vez establecida la resecabilidad del tumor deberá valorarse la operabilidad del mismo en función del estado del paciente.
For the diagnosis of esophagogastric junction (EGJ) tumors, correct anamnesis, physical examination and endoscopic biopsy are required. The classic clinical presentation is progressive dysphagia to solids of short duration in a middle-aged male, sometimes with a history of gastroesophageal reflux, hiatal hernia or a diagnosis of Barrett's esophagus. Upon physical examination, these patients are usually overweight individuals who may present comorbidities associated with the Western lifestyle (metabolic syndrome, obesity), showing no significant weight loss in many cases.1
Preoperative clinical staging is critical in order to select those patients whose disease is localized and could benefit from surgery with curative intent. Ideally, said staging should predict tumor invasion, lymph node involvement and distant metastasis. With clinical TNM staging (cTNM), we are able to select patients who are candidates for endoscopic resection or radical surgery, while avoiding these in patients with distant metastasis.2
The lack of a clear definition for EGJ tumors has made it difficult to topographically classify these tumors. While in the majority of cases it is easy to classify tumors based on endoscopy, barium swallow, computed tomography (CT) or positron-emission tomography (PET), classification can occasionally be complex in locally advanced tumors that obliterate the limits of the EGJ. In any case, all EGJ tumors share certain morphologic characteristics and have a similar prognosis, but there are differences in terms of epidemiology, etiology, distribution and lymph dissemination pattern, which would entail different surgical strategies.
Siewert I tumors have an epidemiology and histology that are similar to adenocarcinomas of the distal esophagus: predominance of males, a general history of gastroesophageal reflux, predominance of the intestinal histologic type (Lauren), and prior presence of Barrett's esophagus. Siewert III tumors are similar to gastric tumors, with a similar proportion of the diffuse intestinal histologic type, and absence of reflux symptoms. The presence of Helicobacter pylori and atrophic gastritis is also associated. The predominance of males is not as notable as in Siewert I.3 Siewert II tumors do not follow such a defined pattern.
The purpose of this study is to review the evidence available about the use and effectiveness of these tests in the clinical study and staging of EGJ tumors.
MethodsReview of the literature restricted to a search of the last 10 years using the PubMed search engine, crossing-referencing the terms “esophagogastric junction” with “cancer”, “tumor”, “adenocarcinoma”, “diagnosis”, “staging”, “complementary tests”, “preoperative examinations”, review of UpToDate review, international clinical guides like NCCN and textbooks, such as Sleisenger and Cirugía esofagogástrica (clinical guidelines of the Spanish Association of Surgeons, AEC).
ResultsFor the initial diagnosis of adenocarcinomas of the EGJ, endoscopic biopsy is required. After histological confirmation, clinical staging is done, which is crucial to define an appropriate multimodal strategy. Necessary complementary tests include: thoracoabdominal-pelvic CT, endoscopic ultrasound (EUS) and PET or PET/CT (both integrated). Other complementary examinations that may be useful are: lab work-up, barium swallow, endoscopic mucosal resection (EMR) or submucosal dissection (ESD) (for assessment or treatment of initial stages) and staging laparoscopy or thoracoscopy.1
Esophagogastroduodenoscopy and tumor biopsy are key studies for the diagnosis of EGJ tumors, with a sensitivity of 96%.4 The endoscopist documents the location of the tumor from the dental arch and the EGJ, tumor length, circumferential involvement, degree of obstruction and presence of Barrett's esophagus. Generally, endoscopy can also define the type of EGJ tumor (Siewert I, II or III), although the presence of a hiatal hernia or diaphragm movements during breathing can make this difficult.5 Certain endoscopic techniques will allow for more detailed study of areas of dysplasia or malignancy. Conventional chromoendoscopy uses staining (lugol, methylene blue, acetic acid or indigo carmine) that enhance subtle changes in the mucosa for targeted biopsies.6,7 Electronic chromoendoscopy has the same objective, using different light filters. Narrow-band imaging increases sensitivity in the detection of dysplasia in Barrett's esophagus from 85% to 92%,8 as well as the negative predictive value from 91% to 94%.9 Endoscopic biopsy as an assessment of the response to neoadjuvant therapy offers poor results (with a sensitivity and negative predictive value of 23%), so it is therefore not recommended.1
After histological confirmation, CT of the chest, abdomen and pelvis may be the technique of choice to rule out metastatic disease.1 CT detects distant metastasis, tumor infiltration of neighboring organs, lymph node involvement, possible esophagobronchial fistula, and also provides information on the status of the pulmonary and hepatic parenchyma.8 CT offers a sensitivity and specificity of 52% and 91%, respectively, while PET offers 71% and 93%, respectively.8
PET and PET/CT are able to increase the sensitivity in the detection of distant metastases by 20% compared to CT,1 and they can detect small tumor metastases (less than 1cm). They are not useful for determining T and limited for N, as the uptake of the peritumoral lymph nodes and the primary tumor can merge in the image, making assessment difficult.8 In some hospitals, PET/CT is performed without intravenous contrast, which may compromise the finding of small metastases. In this case, we should also have a CT scan with intravenous contrast.10 PET and PET/CT may also have a role in re-staging after neoadjuvant therapy in order to define tumors that are responsive to chemotherapy induction treatment.11 “Responders” are defined as patients who have a reduction in metabolic activity greater than 35%, although whether this may affect the prognosis is controversial.12–14
EUS is able to study tumor depth. It can distinguish T1 tumors from the rest, which is essential for selecting candidates for endoscopic or surgical treatment. It has a sensitivity and specificity of 81.6% and 99.4%, respectively, for T1; 81.4% and 96.3% for T2; 91.4% and 94.4% for T3; and 92.4% and 97.4% for T4.15,16 Areas of regional (cN) and non-regional (cM) lymphatic drainage should be studied routinely. This includes the peritumoral, paratracheal, subcarinal, crural, celiac trunk, splenic vein, portacaval and gastrohepatic ligament areas. Correct staging should document the number and location of pathological lymph nodes and categorize them following the criteria of the AJCC (American Joint Committee on Cancer): N0 (no suspicious nodes), N1 (1–2 pathological lymph nodes), N2 (3–6 pathological lymph nodes), N3 (>6 pathological lymph nodes).10 EUS also provides for the study of liver metastases close to the lesser curvature. The presence of perigastric ascites raises the suspicion of carcinomatosis in the absence of other justifying causes.17 Precision for the evaluation of T reaches 90%, while for N it is around 80%, which can increase to 92%–98% when fine-needle aspiration (FNA) is added for lymph node cytology.5 FNA should be reserved for taking biopsies of those nodes that would change the therapeutic approach.18 Stenosing lesions are a limitation for the endoscopic ultrasound study, since the study of only the proximal margin of the stenosis reduces the accuracy to less than 50%. The tumor should not be dilated for better staging.18 However, stenosing tumors that do not allow for endoscopic ultrasound study have a high probability of being locally advanced tumors. Tumors larger than 5cm in length are predictive of T3 with a sensitivity of 89% and specificity of 92%.1 Some studies attribute EUS with greater sensitivity for the study of N compared to CT and PET,19 but this test is not a good technique to evaluate the response to neoadjuvant therapy as it tends to overstage the T when there are inflammatory phenomena, and its accuracy drops to 50% in this situation.2 It is incapable of differentiating between tumor tissue and fibrosis or inflammation, and it is complicated to correctly assess lymphadenopathies after neoadjuvant treatment due to the alterations that occur in their ultrasound appearance.1
As for staging laparoscopy, there is no consensus on its use. The broadest recommendation is established in types II and III adenocarcinomas of the EGJ, T3-4, N+ when neoadjuvant treatment is planned, or in the case of suspected peritoneal carcinomatosis due to indirect signs in other diagnostic techniques.2 Diagnostic or staging laparoscopy should inspect the entire peritoneal cavity to rule out peritoneal or hepatic metastases. It allows the EGJ to be explored to assess the extension of the tumor and to perform a cytology of the peritoneal lavage.5 Diagnostic laparoscopy can change the therapeutic approach in 10%–17% of patients. However, laparoscopic ultrasound does not improve staging versus laparoscopy alone.1
Barium swallow is not an essential or routine test for the diagnosis or staging of EGJ tumors, but it can be useful for assessing the size and obstruction caused by the tumor, while helping to locate it for correct classification as Siewert I, II or III. This test has special relevance in stenosing tumors when the endoscope cannot clear the tumor.2 It may also be useful for the study of esophagobronchial fistulae. In these cases, it is important to use barium sulfate and not a water-soluble hyperosmolar agent to avoid the risk of acute lung edema and pneumonitis.8 EMR and ESD are complementary techniques to the EUS that are able to study the depth of invasion, degree of differentiation and angiolymphatic invasion by resecting the tumor to the mucosa (EMR) or submucosa (ESD). When the margins are free and no factors for a poor prognosis are found, resection can be curative and avoid major surgery.8 EUS does not distinguish reliably between Tis and T1. In these cases, EMR or ESD are essential for correct histological study.20
Magnetic resonance imaging (MRI) is not a routinely used technique for the staging of EGJ tumors. In general, it does not improve the results of CT, but, after the latest technical advances with MRI, some studies have shown improvements in the evaluation of the T and N.10 It can also be useful for the study of liver metastases.2
In addition, once the resectability of the tumor has been established, the specific operability of the tumor must be assessed in terms of age, respiratory function, performance status, cardiovascular function, liver function and nutritional status.21
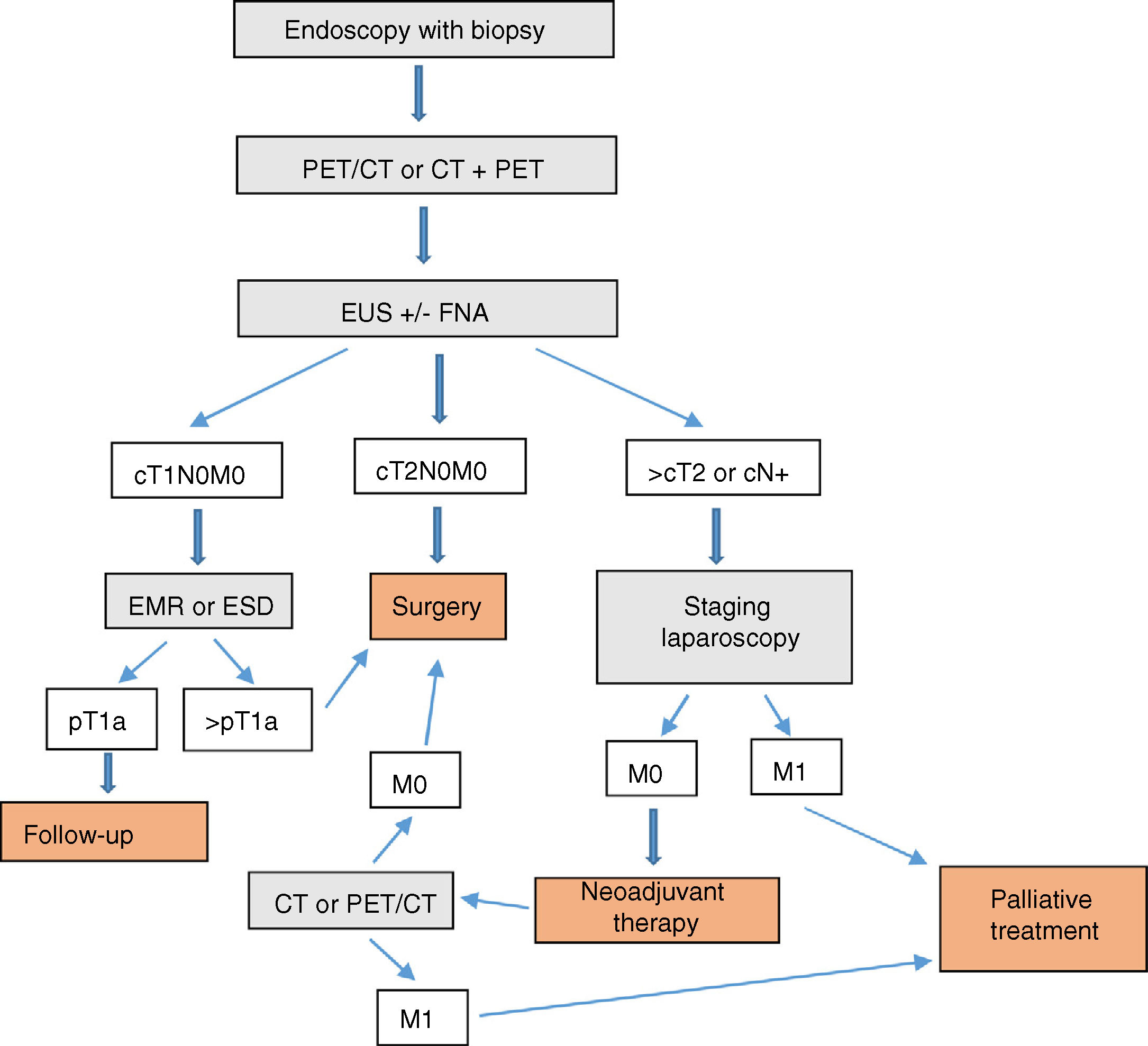
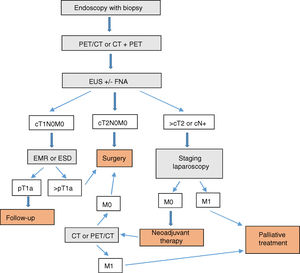
ConclusionIn our opinion, the complementary studies that should be carried out to correctly diagnose and stage EGJ tumors include (Fig. 1):
- 1.
Endoscopy with biopsy
- 2.
PET/CT or CT+PET (according to availability)
- 3.
EUS±FNA
- 4.
Barium swallow (optional)
In T1N0M0 tumors, EMR or ESD should be considered part of either the diagnosis or even the treatment. In EGJ tumors that are Siewert II–III>T2 or N+, staging laparoscopy should be assessed.
Conflict of InterestsNone.
The authors would like to thank Pere Rebasa and Sandra Montmany.
Please cite this article as: Luna Aufroy A, Navarro Soto S. Pruebas diagnósticas empleadas en la estadificación preoperatoria del cáncer de la unión esofagogástrica: rendimiento y recomendaciones basadas en la evidencia. Cir Esp. 2019;97:427–431.