The widespread use of diagnostic imaging techniques has led to an increase in the identification of hepatic masses, making it necessary to differentiate between malignant and benign masses. For this purpose, the technique of choice continues to be abdominal magnetic resonance imaging (MRI), although occasionally there is persisting doubt between metastases and hemangiomas.
Hepatic hemangiomas are the most frequent benign liver tumors. They are usually asymptomatic, although large hemangiomas may cause abdominal pain. 99mTc-labeled red blood cell scintigraphy is a non-invasive technique that is able to differentiate hemangiomas from other space-occupying lesions (SOL) when there are clinical-radiological discrepancies.1,2 Likewise, somatostatin receptor scintigraphy is able to characterize the liver parenchyma in patients with a history of neuroendocrine tumors. In this way, functional information from nuclear medicine techniques is of interest to establish the precise diagnosis of hepatic SOL of uncertain etiology.
In this paper, we present the case of a patient diagnosed with neuroendocrine cancer, discussing how nuclear medicine techniques with labeled red blood cells and somatostatin analogs (octreotide) were able to make a definitive diagnosis and guide the therapeutic approach.
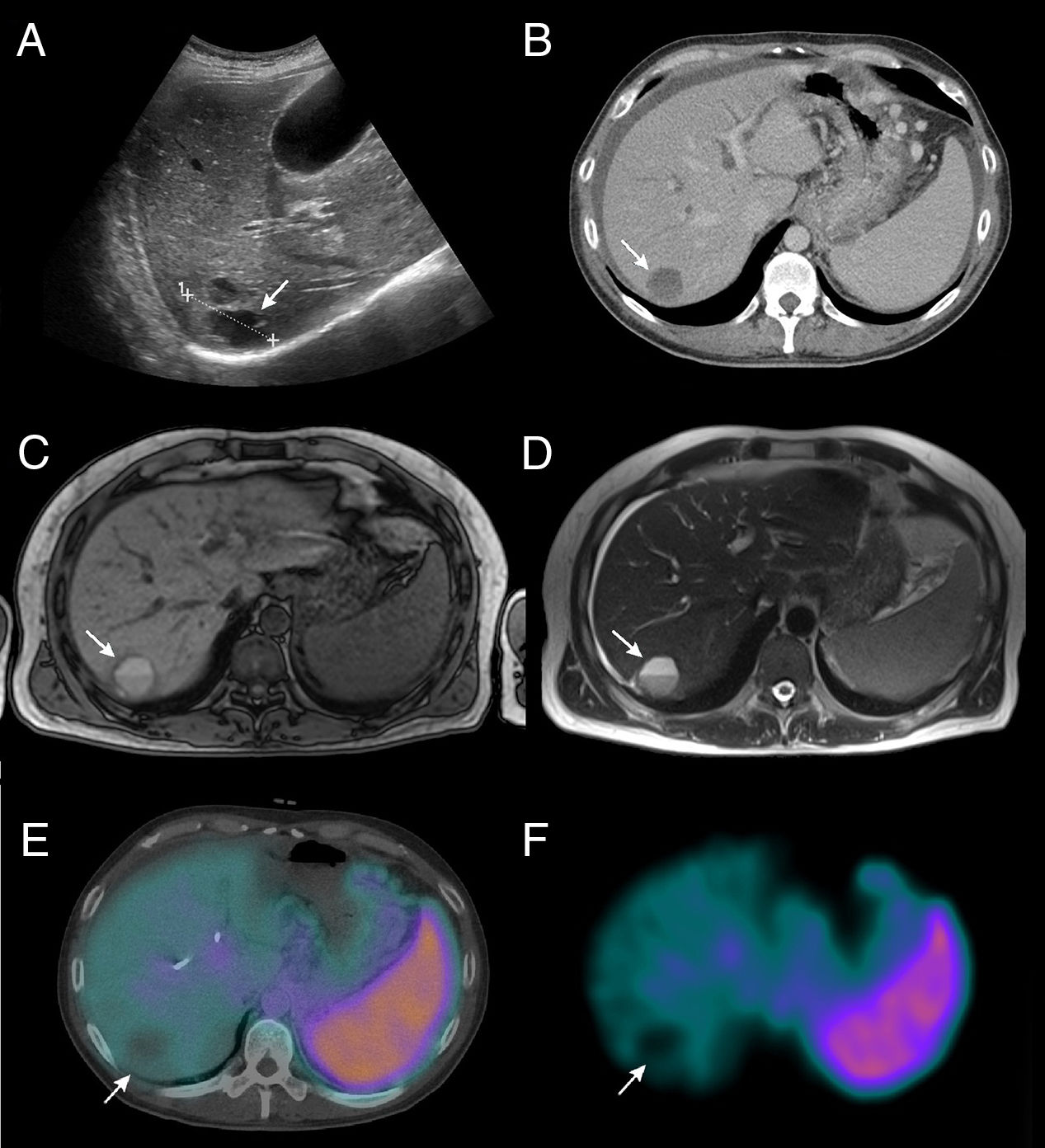
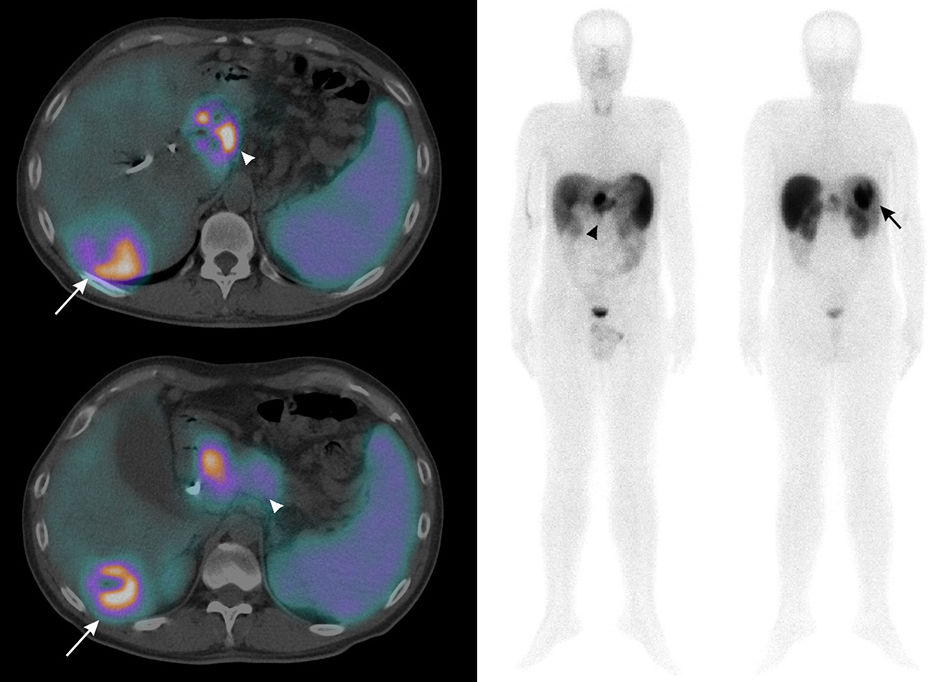
Clinical case: A 43-year-old man was being studied for abdominal pain, and ultrasound detected a mass in the body/tail of the pancreas (Fig. 1A). The anatomic pathology diagnosis was non-functioning neuroendocrine tumor. On ultrasound and in the extension study with CT (Fig. 1B) and MRI (Figs. 1C and D), a hypervascular SOL was also observed in liver segments VI-VII, and there were doubts between the diagnoses of metastasis, hemangioma or hydatid cyst. In this clinical context, and to perform the differential diagnosis of hemangioma versus possible metastatic lesion, a study was done with 99mTc-labeled red blood cells, which showed scant tracer uptake by the hepatic lesion under study (Fig.s 1E and F, arrow), ruling out the possibility of hemangioma.1 Additionally, 99mTc-octreotide scintigraphy detected a high-intensity tracer deposit in the head of the pancreas (Fig. 2, arrowhead). Regarding the liver parenchyma, a mass with hyperenhancement was observed in segment VI–VII with a central area of hypoenhancement (Fig. 2, arrow). These findings were suggestive of a neuroendocrine tumor of the head of the pancreas with a solitary hepatic metastasis.
(A) Abdominal ultrasound showing a well-defined, solid nodular image measuring 4.5cm in diameter in the liver parenchyma (arrow); (B) abdominal CT scan showing hypodense hepatic lesion (arrow) with hypervascular behavior and little enhancement, solid-cystic characteristics at the lower end, and levels inside, suggesting that it is a hypervascular SOL with rapid growth and necrosis, compatible with a hydatid cyst, abscess, hemangioma or metastasis; (C and D) MRI in sequences T1 and T2, respectively, which highlight a nodular lesion (arrow) with cystic-necrotic areas and irregular contrast uptake that presents portal lavage; also the size has increased compared to previous tests; (E and F) SPECT/CT and SPECT, respectively, with 99mTc-marked red blood cells of the abdomen showing an absence of tracer uptake compared to the hepatic lesion being studied (arrow).
SPECT/CT (left) and complete body (right) of 99mTc-octreotide showing an epigastric mass with increased heterogeneous uptake of somatostatin analogs, correlating with the mass in the body and tail of the pancreas that had been observed in previous tests (arrowhead). Regarding hepatic nodular lesion, hyperuptake was seen in the peripheral halo (arrow), with absence of uptake enhancement, probably related to areas of central necrosis, being therefore compatible with metastasis of a primary pancreatic neuroendocrine tumor.
Neuroendocrine tumors (NET) are a rare and heterogeneous group of tumors that mainly affect the gastroenteropancreatic and bronchopulmonary systems. Gastroenteropancreatic neuroendocrine tumors (GEP-NET), as in this case that we present, settle more frequently in the small intestine (30.8%), followed by the rectum (26.3%), large intestine (17.6%), pancreas (12.1%), stomach (8.9%) and appendix (5.7%).3
The secondary symptoms of functioning GEP-NET are due to the hypersecretion of the substances that they produce, store and secrete (serotonin, insulin, glucagon, gastrin, VIP, somatostatin), while the non-functioning ones will cause late symptoms due to a mass effect as they do not produce these substances.3
The diagnosis and/or follow-up of GEP-NET are carried out by determining biochemical markers. These include general markers, such as chromogranin A (CgA) and pancreatic polypeptide (PP), or specific markers according to the hormone that the tumor secretes, thus providing a more specific follow-up.
The imaging techniques available for the diagnosis of these tumors are CT scan for the initial study and follow-up, MRI as the most sensitive technique for the assessment of liver lesions, and ultrasound to guide biopsy sampling.
In addition, nuclear medicine techniques with somatostatin analogs (octreotide) labeled with 99mTc or 111In are considered fundamental for the study of NET that overexpress somatostatin receptors (SSTR), and type 2 SSTR are especially important. The somatostatin analog (octreotide) technique is highly sensitive (70%–90%) and useful for the assessment of primary tumors, possible metastatic involvement and to make correct therapeutic decisions, since a positive study predicts a good response to treatment with somatostatin analogs.4
The only curative treatment of resectable NET is radical surgery, hence the importance of an accurate and early diagnosis. For more advanced stages, there are multiple different therapeutic options, such as somatostatin analogs, interferon (IFN), chemotherapy, monoclonal antibodies and therapy with radionuclide-labeled peptides.
In conclusion, scintigraphy techniques with different radiotracers, such as those described in this article, accurately define relevant metabolic aspects of hepatic lesions that are of interest in the differential diagnosis of unidentified SOL of the liver. Furthermore, they correctly establish neuroendocrine tumor stages.1–5
Please cite this article as: León-Asuero-Moreno I, Calvo-Morón MC, Garcia-Gomez FJ, Sabatel-Hernández G, Castro-Montaño J. Diagnóstico diferencial de masa hepática mediante gammagrafía con hematíes marcados y octreótido. Cir Esp. 2019;97:355–357.