Division of the thoracic sympathetic chain is the standard treatment for severe palmar and/or axillary hyperhidrosis and facial flushing. Clipping is an alternative option which allows the block to be reverted in cases of intolerable compensatory sweating.
MethodsThis is a prospective study performed to assess: (a) results of clipping of the thoracic sympathetic chain in patients with palmar and/or axillary hyperhidrosis and facial flushing; and (b) to determine the improvement obtained after removal of the clip in patients with unbearable compensatory sweating. We included 299 patients (598 procedures) diagnosed with palmar hyperhidrosis (n=110), palmar and/or axillary hyperhidrosis (n=78), axillary hyperhidrosis (n=35), and facial flushing (n=76), who underwent videothoracoscopic clipping between 2007 and 2015.
Results128 men and 171 women were treated, with mean age of 28 years. A total of 290 patients (97.0%) were discharged within 24h. The procedure was effective in 92.3% (99.1% in palmar hyperhidrosis, 96.1% in palmar and/or axillary hyperhidrosis, 74.3% in axillary hyperhidrosis, and 86.8% in facial flushing). Nine patients (3%) presented minor complications. Compensatory sweating developed in 137 patients (45.8%): moderate in 113 (37.8%), severe in 16 (5.3%) and unbearable in 8 (2.7%). The clip was removed in these 8 patients; symptoms improved in 5 (62.8%), with sustained effect on hyperhidrosis in 4 of them.
ConclusionsClipping of the thoracic sympathetic chain is an effective and safe procedure. If incapacitating compensatory sweating develops, this technique allows the clips to be removed with reversion of symptoms in a considerable number of patients.
El tratamiento estándar de la hiperhidrosis palmar o axilar y el rubor facial es la sección de la cadena simpática torácica. La interrupción de la transmisión nerviosa de la cadena simpática torácica bilateral con clip, conocida como «pinzamiento», constituye una alternativa con la opción de revertir el procedimiento en caso de sudoración compensatoria intolerable.
MétodosEstudio prospectivo para evaluar: a) resultados del «pinzamiento» en pacientes con hiperhidrosis palmar o axilar y rubor facial, y b) determinar la mejoría obtenida tras la retirada del clip en pacientes con sudoración compensatoria intolerable Se incluyó a 299 pacientes (598 procedimientos) diagnosticados de hiperhidrosis palmar (n = 110), palmar o axilar (n = 78), axilar (n = 35) y rubor facial (n = 76), tratados mediante «pinzamiento» videotoracoscópico de 2007 a 2015.
ResultadosCiento veintiocho varones y 171 mujeres con una edad media de 28 años. En 290 casos (97,0%) pudo darse el alta dentro de las primeras 24h. En un 92,3% el procedimiento fue efectivo (99,1% hiperhidrosis palmar, 96,1% hiperhidrosis palmar o axilar, 74,3% hiperhidrosis axilar y 86,8% rubor facial). Nueve pacientes (3%) presentaron complicaciones menores. En 137 pacientes (45,8%) apareció sudoración compensatoria: moderada 113 (37,8%), severa 16 (5,3%) e intolerable 8 (2,7%). Se retiró el clip en estos 8 pacientes, mejorando los síntomas en 5 (62,8%), con efecto mantenido sobre la hiperhidrosis en 4 de ellos.
ConclusionesEl «pinzamiento» de la cadena simpática torácica es una técnica efectiva y segura. En caso de sudoración compensatoria incapacitante, esta técnica permite la retirada del clip y la reversión de los síntomas en un porcentaje notable de pacientes.
Essential or primary hyperhidrosis is a disorder characterized by excessive palmar, axillary or plantar sweating, or a combination of these or other bodily regions to varying degrees. It is estimated that between 1% and 3% of the population is affected.1 Facial flushing consists of uncontrolled reddening of the face due to inappropriate dilatation of the facial blood vessels. Both hyperhidrosis and facial flushing are caused by overstimulation of the sympathetic nervous system. Currently, when dermatological therapies have been ineffective, the most standardized treatment providing the best result for both cases is bilateral endoscopic thoracic sympathicotomy. This surgical procedure involves interruption of the thoracic sympathetic nerve at different levels depending on the disorder being treated, thereby achieving the disappearance of hyperhidrosis and facial flushing.2 This technique has been shown to be safe and effective.3 Its most frequent side effect (30%–75% of cases) is compensatory sweating, which is excessive postoperative sweating on the back, lower limbs and abdomen.4 This symptom is almost always subtle and well tolerated by patients, but in some people (5%) it can be quite abundant and problematic, especially during physical exercise and conditions of very high temperatures. In these cases, the patient may request “reversal” of the sympathicotomy to alleviate the compensatory sweating, even if this means returning to the symptoms prior to the intervention. In this context, an alternative technique to sympathicotomy has arisen, involving interruption of the nerve transmission of the bilateral thoracic sympathetic chain with video-assisted thoracoscopy, also known as “clipping”.5 This procedure is capable of treating hyperhidrosis and facial flushing, can resolve problematic compensatory sweating, and its results and complications are comparable to standard sympathicotomy.6
In 2013, Martínez-Barenys et al.7 published the first results of video-assisted thoracoscopic clipping in 44 patients (88 procedures), with a hyperhidrosis recurrence rate of 4.5% and an incidence of compensatory sweating of 65.9%. The objectives of the present study were: (a) to evaluate the results of clipping the thoracic sympathetic chain by video-assisted thoracoscopy in an extensive case series of 299 patients with palmar or axillary hyperhidrosis and facial flushing; and (b) to determine the improvement obtained after the removal of the clip in patients with intolerable compensatory sweating.
MethodsFrom January 2007 to December 2015, all patients diagnosed consecutively with hyperhidrosis or facial flushing with indication for surgical treatment were included in a prospective study. The preoperative evaluation included complete patient medical history, complete physical examination, chest X-ray, complete blood count and thyroid function tests in case of suspicion of thyroid disease. The surgical indication was based on the following criteria: (a) confirmation through the preoperative interview that the disorder had a notable impact on the patient's daily life, both at work and socially; (b) exclusion of other underlying causes through the clinical history and the results of the complementary studies; and (c) confirmation of the lack of effectiveness of the previous conservative treatments. The hospital Ethics Committee approved the study, and all patients gave their written consent.
All operations were performed under general anesthesia with selective ventilation and in semi-Fowler position, with both arms in hyperextension. A digital thermometer was routinely used to measure the palmar temperature, thereby functioning as a guide to verify the correct sympathetic blockade. The instrumentation used included two 10-mm ports, a 10mm endocamera with 30° vision, a diathermy hook and an angled clip applicator (Acuclip™ Right-Angle Multiple Clip Applier, Covidien/Medtronic, Cornellá del Llobregat, Barcelona, Spain), with 20 titanium 8mm clips. Two 10-mm incisions were made for ports in the 3rd and 5th intercostal spaces, on the mid- and anterior axillary lines, respectively. Once the sympathetic nerve was identified, the appropriate level for the placement of the clip was found by counting the ribs (R2: 2nd rib, R3: 3rd rib, R4: 4th rib and R5: 5th rib). Under endoscopic control, the parietal pleura was opened at the desired level using the electrocoagulation hook.6 We carried out a sympathetic block by clipping the sympathetic nerve at the R2 level for facial flushing, R3–R4 for palmar hyperhidrosis and R4–R5 for palmar and axillary hyperhidrosis. When Kuntz nerves were observed, they were electrocoagulated. After the complete release of the sympathetic nerve and having created sufficient space, the clip applicator was inserted through the upper trocar. The rib under the sympathetic nerve made it difficult for the applicator to pass, so this had to be done through the upper or lower intercostal space. The head of the applicator was carefully pressed into the space and advanced laterally until the mouth of the U-shaped device completely encompassed the sympathetic nerve, at which point the clip was placed horizontally.6 We used palmar temperature increase and changes in the pulse wave as indicators of the correct blockade of the sympathetic nerve. The technique was considered effective when, in addition to the surgeon visually verifying the complete compression of the sympathetic nerve by the clip, the anesthesiologist verified: (a) increase in digital temperature (regardless of the exact increase); and (b) increase in the pulse wave (any increase). In the contralateral hemithorax, the same procedure was performed. At the end of the operation, chest X-rays were taken to rule out pneumothorax.
In the event of massive compensatory sweating, the clips were able to be easily removed. The incision scars of the previous surgery were used for the ports, the camera was inserted and directed toward the lung apex to achieve better visibility of the sympathetic nerve. If pleural adhesions appeared, we were able to release them. Depending on the time elapsed since the first surgery, it was possible that a layer of fibrosis may have covered the clip, but, regardless of the thickness of the layer, the use of the ribs as reference points facilitated the localization of the sympathetic nerve and the clip. To remove it, a curved endo-instrument was used, the tip of which grasped the ‘tail’ of the clip (proximal end) and, with a smooth lateral movement, the clip was able to be easily separated from the nerve.8
A visual analog scale (VAS) was used to determine the degree of patient satisfaction with the results of the surgery (VAS 9–10, very satisfied; VAS 6–8, satisfied; VAS 3–5, dissatisfied; VAS 0–2, very unsatisfied). VAS categories 6–10 were considered satisfied with the surgery due to the disappearance or significant improvement of the flushing or anhidrosis of the affected area (effective procedure), and VAS categories 0–5 were considered dissatisfaction due to the persistence of flushing or hyperhidrosis (ineffective procedure). Compensatory sweating was assessed by the answer ‘yes’ or ‘no’ to the question of having experienced abnormal excessive sweating in other parts of the body after surgery. If the answer was affirmative, the patient classified it as ‘moderate’ (localized, without interfering with the activities of daily living), ‘severe’ (systemic, tolerably affecting daily life) or ‘intolerable’ (systemic, significantly affecting daily life).
Likewise, given that the patients were part of the ambulatory surgery program, the nurses of the minimally invasive surgery unit collected the degree of postoperative pain. A VAS (0–10) was used to define the intensity of pain as mild or mild-moderate (VAS <4), moderate-severe (VAS 4–6) or very intense (VAS >6). Patients could be discharged early from hospital as part of the program if the following criteria were met: tolerance to oral fluid intake, ability to stand and walk accompanied, pain intensity measured by a VAS of ≤3 and absence of complications.
Patient data were collected prospectively with a questionnaire specifically designed for the study (annex, supplementary material) and analyzed retrospectively, with a minimum follow-up of 12 months. A descriptive analysis of the results is presented.
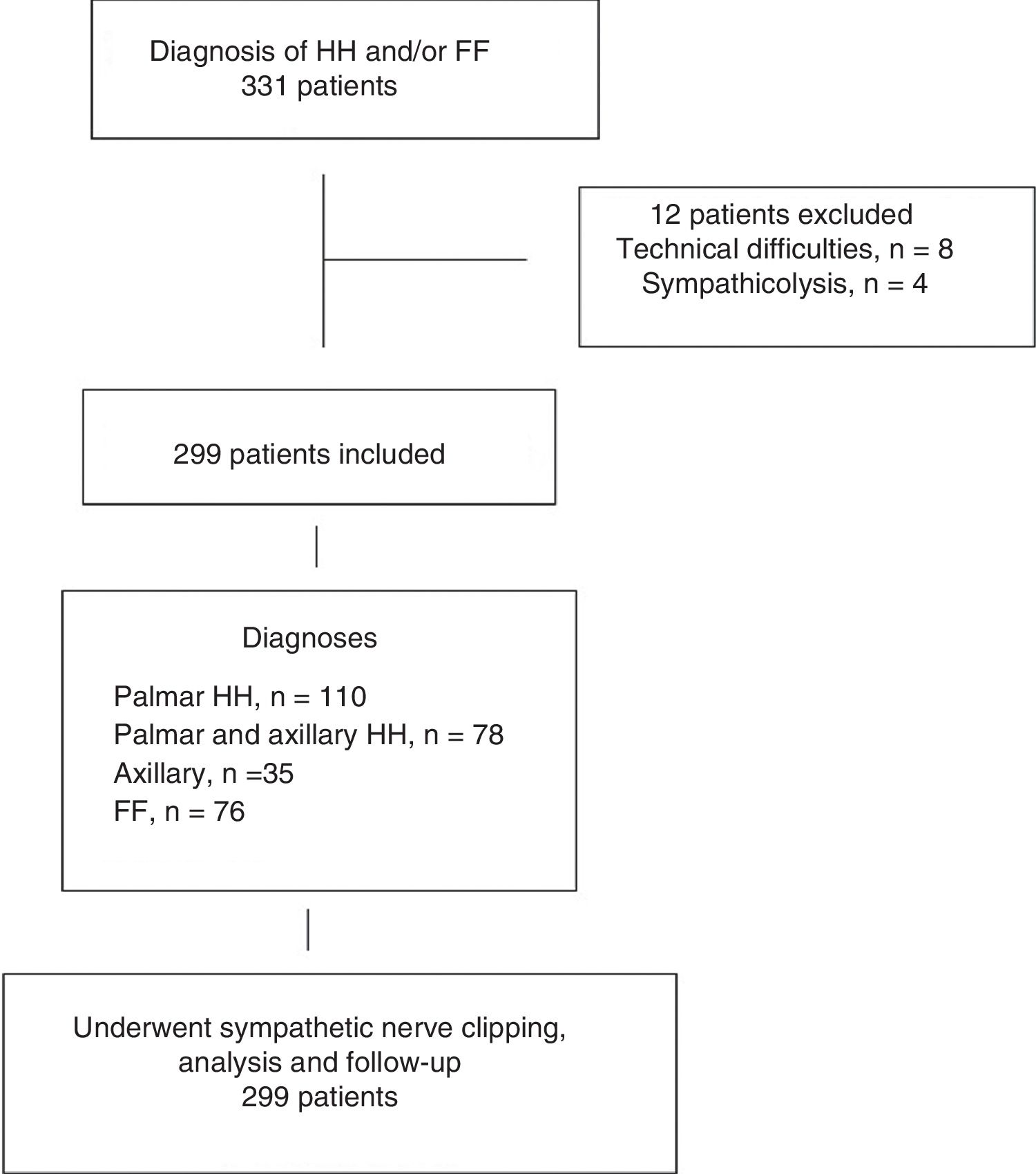
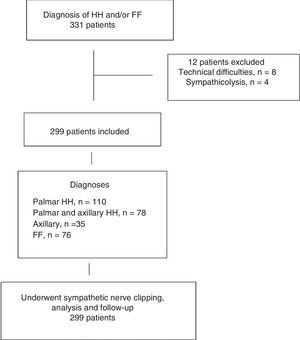
ResultsOut of the 331 patients who were candidates for sympathetic clipping, 32 were excluded: 23 due to technical difficulties (learning curve) and 4 who preferred the classical technique of sympathicolysis. The distribution of patients is summarized in Fig. 1. The study population consisted of 299 patients (128 men, 171 women), with a mean age of 28 years (range 15–65). In 76 patients, the main symptom was facial flushing (2 patients had been unsuccessfully treated surgically at another hospital), in 110 palmar hyperhidrosis, in 78 palmar and axillary hyperhidrosis, and in the remaining 35, pure axillary hyperhidrosis. The baseline patient characteristics are shown in Table 1.
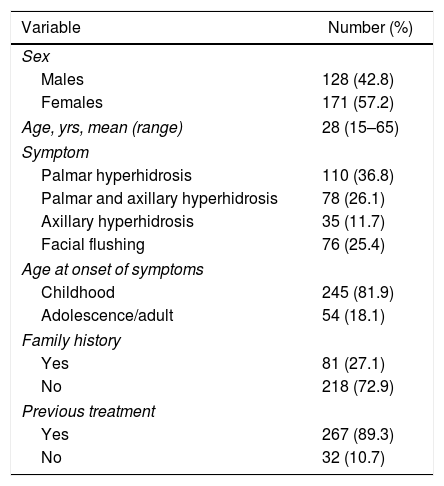
Baseline Characteristics of the 299 Patients Included in the Study.
| Variable | Number (%) |
|---|---|
| Sex | |
| Males | 128 (42.8) |
| Females | 171 (57.2) |
| Age, yrs, mean (range) | 28 (15–65) |
| Symptom | |
| Palmar hyperhidrosis | 110 (36.8) |
| Palmar and axillary hyperhidrosis | 78 (26.1) |
| Axillary hyperhidrosis | 35 (11.7) |
| Facial flushing | 76 (25.4) |
| Age at onset of symptoms | |
| Childhood | 245 (81.9) |
| Adolescence/adult | 54 (18.1) |
| Family history | |
| Yes | 81 (27.1) |
| No | 218 (72.9) |
| Previous treatment | |
| Yes | 267 (89.3) |
| No | 32 (10.7) |
Data expressed as frequencies and percentages in parentheses, unless otherwise indicated.
In 290 cases (97.0%), discharge occurred within 24h of surgery, and 249 (83.3%) were part of a minimally invasive ambulatory surgery program. The main results are summarized in Table 2. In 92.3% of the patients, the symptoms improved. By disease, the percentages of improvement were 99.1% for the group of patients with palmar hyperhidrosis, 96.1% for the palmar or axillary hyperhidrosis group, 74.3% for the pure axillary hyperhidrosis group, and 86.8% for the facial flushing group.
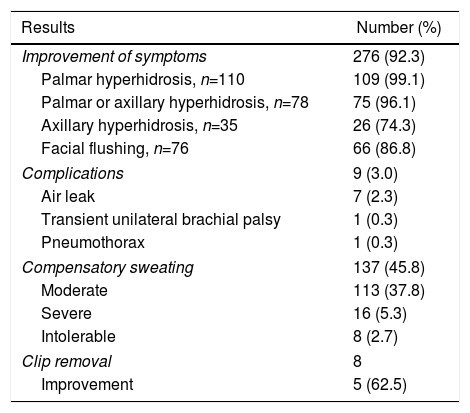
Results in 299 Patients Treated With Bilateral Clipping of the Thoracic Sympathetic Chain.
| Results | Number (%) |
|---|---|
| Improvement of symptoms | 276 (92.3) |
| Palmar hyperhidrosis, n=110 | 109 (99.1) |
| Palmar or axillary hyperhidrosis, n=78 | 75 (96.1) |
| Axillary hyperhidrosis, n=35 | 26 (74.3) |
| Facial flushing, n=76 | 66 (86.8) |
| Complications | 9 (3.0) |
| Air leak | 7 (2.3) |
| Transient unilateral brachial palsy | 1 (0.3) |
| Pneumothorax | 1 (0.3) |
| Compensatory sweating | 137 (45.8) |
| Moderate | 113 (37.8) |
| Severe | 16 (5.3) |
| Intolerable | 8 (2.7) |
| Clip removal | 8 |
| Improvement | 5 (62.5) |
Nine patients (3%) presented complications that were grade I of the Clavien–Dindo classification in 8 cases and grade III in one case of delayed pneumothorax requiring chest drainage. Seven patients were admitted during the first 24h due to air leak, one patient because of transient unilateral brachial plexus palsy and the remaining patient was readmitted 72h after surgery for late-onset pneumothorax that required pleural drainage. In 4 patients (1.3%), pneumothorax of the apical lamina was found on the postoperative chest radiograph without clinical translation, having resolved spontaneously on the follow-up study a week after surgery.
In 137 patients (45.8%), compensatory sweating appeared, which was classified as ‘severe’ in 16 (5.3%) and ‘intolerable’ in 8 (2.7%). These 8 patients requested the withdrawal of the clip because sweating seriously affected their daily activities.9 In these cases, the intervention was performed 2 months after sympathetic clipping in 3 patients, 3 months after in 2 patients, 7 months after one, 14 months after in one, and 3 years after in one. In 3 cases, there were technical difficulties due to firm pleural adhesions in the interventions performed after 7 and 14 months and 3 years. After the withdrawal of the clip, compensatory sweating improved in 5 of the 8 patients (62.5%), and these 5 had been treated within the first 3 months of the initial operation; the effect on hyperhidrosis was maintained in 4 of them.
DiscussionThe treatment of hyperhidrosis and facial flushing varies from medical dermatological therapy to surgery. Medical treatments include aluminum chloride hexahydrate, oral anticholinergics, iontophoresis, and intradermal injection of botulinum toxin.10 These disorders generate a high degree of anxiety, affecting patients both personally and in the work environment, so when conservative procedures fail, thoracic endoscopic sympathicolysis is indicated.11 The surgical approach has evolved from an open approach that required hospitalization to the current video-assisted thoracoscopic approach that can be carried out as ambulatory surgery.12,13 In our department, we have done sympathectomy using 2 trocars since the beginning of the clipping program 10 years ago. Previously, we had performed sympathectomy with a single trocar. The degrees of pain in both series of patients have made it possible to include these procedures (before and after the implementation of clips) in an ambulatory minimally invasive surgery program, without no observed greater intensity of pain with the change to 2 incisions that would prevent early discharge.
Different techniques for endothoracic sympathicolysis have been described for the treatment of hyperhidrosis and facial flushing.14,15 The most common technique is sympathicotomy, which entails identifying the sympathetic chain and transecting it, using an electronic or harmonic scalpel.16–18 Despite its effectiveness and safety, this procedure has the disadvantage of being irreversible. After interruption, nerve conduction by the sympathetic nerve is difficult to recover and the risk of massive compensatory sweating persists. Thus, in the event of this complication, it is possible that patients may prefer to have continued suffering from the original hyperhidrosis or facial flushing.
Although intolerable compensatory sweating after division of the sympathetic nerve affects approximately 5% of patients, therapeutic options are limited. Local injection of botulinum toxin has been suggested in those areas where sweating is more copious.19 The reconstruction of the sympathetic nerve using the intercostal nerve or the sural nerve has also been proposed, but experiences with this procedure are limited. In 1988, Telaranta20 described a clinical case of palmar hyperhidrosis and reconstruction with a sural nerve graft by open surgery that had satisfactory results one year after the intervention. In 2008, Latif et al.21 used a porcine model to demonstrate that it was feasible to perform an intercostal nerve graft by robotic thoracoscopy. In a series of 19 patients with severe compensatory hyperhidrosis an average of 47 months after primary surgery treated by reconstructive surgery with the intercostal nerve, Haam et al.22 described improved sweating in 9 patients (47.4%) and marked improvement in only 3 patients (15.8%). Recently, Rantanen and Telaranta23 have published the long-term results (mean follow-up 87 months) of 19 patients out of 150 who answered a questionnaire to evaluate the outcome of the reconstruction with intercostal nerve (n=11) or sural nerve (n=8) by compensatory sweating after endoscopic thoracic sympathectomy. The decrease in sweating was significant in 7 (36.8%) patients, moderate in 7 (36.8%) and mild in one (5.3%). In 3 patients, the result was negative (15.8%) and the condition of one patient worsened. Although the percentage of improvement was 73.7%, the low response rate of 12.7% introduces a very important bias in the interpretation of the results. On the other hand, conservative treatments, including weight reduction, topical application of aluminum salts, subcutaneous injection of botulinum toxin and treatment with oral anticholinergics (glycopyrrolate, oxybutynin), are usually ineffective in patients who develop significant compensatory sweating.24
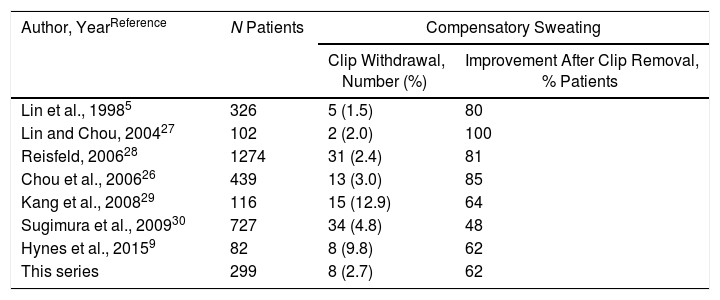
The clipping technique is as effective as the irreversible interruption of the sympathetic chain and is the only procedure, so far, capable of reversing the block in the thoracic sympathetic nervous system.18,25 In 1998, Lin et al.5 introduced clipping of the sympathetic chain at the R2 level in 326 patients with palmar hyperhidrosis with excellent results, which has subsequently been confirmed in different studies.9,26–30 The greatest potential advantage of the clipping technique versus permanent sympathectomy is the withdrawal of the clip if intolerable compensatory sweating develops, which is technically a minimally invasive procedure that requires limited dissection of the area where the clip was inserted. Removal of the clip is simple, and video-assisted thoracoscopy can be repeated through the same access incisions.26 In the main series published in the literature, the percentages of improvement of severe compensatory sweating after clip removal vary between 48% and 100% (Table 3). In the present study, 8 patients (2.7%) required withdrawal of the clip due to intolerable sweating, and the procedure was able to be performed in all. In 5 patients (62.5%), improvement was achieved, and 4 of them maintained the initial effect on hyperhidrosis. It should be noted that the 5 cases in which improvement of the intolerable compensatory sweating was obtained, the withdrawal of the clip was carried out within the first 3 months of the initial operation (3 cases after 2 months and 2 cases after 3 months). In this sense, we concur with Hynes et al.,9 who recommend the withdrawal of the clip as soon as possible - and always within the first 3 months.
Data From the Main Series About Clipping of the Thoracic Sympathetic Chain and Improvement of Compensatory Sweating After Clip Removal.
| Author, YearReference | N Patients | Compensatory Sweating | |
|---|---|---|---|
| Clip Withdrawal, Number (%) | Improvement After Clip Removal, % Patients | ||
| Lin et al., 19985 | 326 | 5 (1.5) | 80 |
| Lin and Chou, 200427 | 102 | 2 (2.0) | 100 |
| Reisfeld, 200628 | 1274 | 31 (2.4) | 81 |
| Chou et al., 200626 | 439 | 13 (3.0) | 85 |
| Kang et al., 200829 | 116 | 15 (12.9) | 64 |
| Sugimura et al., 200930 | 727 | 34 (4.8) | 48 |
| Hynes et al., 20159 | 82 | 8 (9.8) | 62 |
| This series | 299 | 8 (2.7) | 62 |
In an experimental study with a porcine model, Loscertales et al.31 described Wallerian and axonal degeneration 10 days after clipping with little residual myelin and the presence of unmyelinated fibers 20 days after the withdrawal of the clip. Therefore, although these authors question the reversibility of the technique, clinical results demonstrate the feasibility and effectiveness of clip removal with satisfactory results in approximately 70% of cases (Table 3). While the efficacy of clipping and the absence of compensatory sweating are determining factors in the quality of life in these patients,32 the risk factors for the appearance of compensatory sweating have not been studied, although it seems that there is a relationship with the level and the extent of thoracic sympathectomy.32 In the series of 727 patients described by Sugimura et al.,30 clipping at level T3+4 compared to T2 or T2+3 was associated with a greater degree of satisfaction and lower rate of compensatory sweating.
In summary, the results of the present study indicate that clipping the thoracic sympathetic chain by video-assisted thoracoscopy is an effective and safe technique for the treatment of patients with palmar or axillary hyperhidrosis and facial flushing in whom conservative strategies have failed. In addition, in cases of severe compensatory sweating after sympathicotomy, this technique allows for the clip to be withdrawn and the symptoms reversed.
Conflict of InterestsThe authors do not have any conflicts of interests.
The authors would like to thank Dr. Marta Pulido for assisting in the editing and revision of the text.
Please cite this article as: Fibla Alfara JJ, Molins López-Rodó L, Hernández Ferrández J, Guirao Montes Á. Efectividad de la interrupción de la transmisión nerviosa de la cadena simpática torácica bilateral con clip para el tratamiento de la hiperhidrosis palmar y/o axilar severa y el rubor facial. Cir Esp. 2019;97:196–202.