To assess the effectiveness of a protocol for the prevention of surgical site infection (SSI) in colorectal surgery.
Patients and methodsEvaluation of 2 cohorts of patients undergoing colon and rectal surgery in a tertiary public hospital: A historical cohort (2008–2011) and a prospective one (after the implementation of the programme in 2012). The main measures established were: Adequacy of preoperative antimicrobial prophylaxis, maintaining patient normothermia and appropriate glove change during the intervention. Comparability of the two cohorts was determined by a bivariate analysis of age, sex, NNIS index, ASA index, surgical time, perioperative transfusion, diagnosis, diabetes and renal failure.
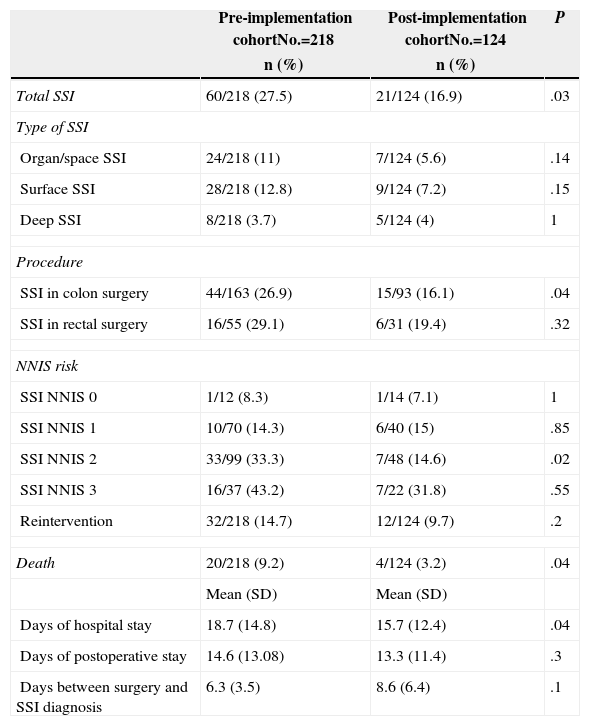
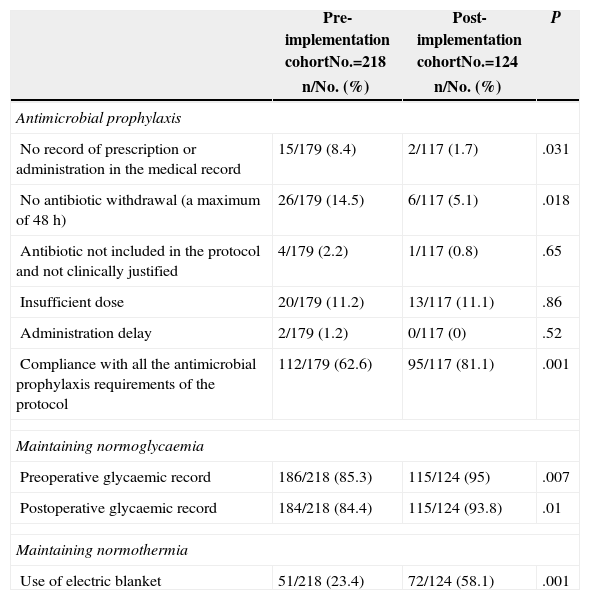
ResultsWe assessed 342 patients (256 underwent colon surgery and 86 rectal surgery), divided into 2 cohorts: prior period (218), and post-implementation period (124). The cumulative incidence of SSI in the first cohort was 27.5% (95% CI, 21.6–33.4), and in the post-intervention cohort 16.9% (95% CI, 10.3–23.5, P=.03). Postoperative mortality was 9.2% (95% CI, 5.4–13) in the first cohort and 3.2% (95% CI, 0.1–6.3) in the post-intervention cohort (P=.04). The inadequacy of prophylaxis decreased from 37.4% (95% CI, 30.4–44.6) to 18.9% (95% CI, 11.9–26.1) (P=.001).
ConclusionA significant decrease in the frequency of SSI, post-surgical mortality and inadequate antimicrobial prophylaxis is verified after the implementation of a protocol in colorectal surgery
Evaluar la efectividad de un protocolo para la prevención de la infección de localización quirúrgica (ILQ) en cirugía colorrectal.
Pacientes y métodosEvaluación de 2 cohortes de pacientes intervenidos de colon y recto en un hospital público de tercer nivel: una cohorte histórica (2008–2011) y otra prospectiva (posterior a la implementación del programa en 2012). Las principales medidas establecidas fueron: adecuación de la profilaxis antimicrobiana prequirúrgica, mantenimiento de la normotermia del paciente en el quirófano y adecuación del cambio de guante durante la intervención. Se determinó la comparabilidad de ambas cohortes mediante un análisis bivariable de la edad, sexo, factores e índices de riesgo (índice NNIS, índice ASA, tiempos quirúrgicos, transfusión periquirúrgica, diagnóstico, diabetes, insuficiencia renal).
ResultadosSe evaluó a 342 pacientes (256 intervenidos de colon y 86 de recto), distribuidos en 2 cohortes: periodo previo (218) y periodo postimplementación del programa (124). La incidencia acumulada de ILQ de la primera cohorte fue del 27,5% (IC 95%=21,6–33,4), y de la cohorte postintervención 16,9% (IC 95%=10,3–23,5; p=0,03). La mortalidad postoperatoria fue del 9,2% (IC 95%=5,4–13) en la primera cohorte y del 3,2% (IC 95%=0,1–6,3) en la cohorte postintervención (p=0,04). La administración inadecuada de la profilaxis disminuyó del 37,4% (IC 95%=30,4–44,6) al 18,9% (IC 95%=11,9–26,1; p=0,001).
ConclusionesTras la implementación de un protocolo para la prevención de la infección quirúrgica en cirugía colorrectal se verifica una disminución significativa de la frecuencia de ILQ, de la mortalidad posquirúrgica y de la profilaxis antimicrobiana inadecuada.
In spite of the improvements in surgical techniques that have been introduced during the last few years, surgical site infections (SSI) continue to be a common complication. It is estimated that slightly over 5% of patients treated with any kind of surgery will suffer a SSI.1,2
In this kind of infection, the highest rates correspond to colorectal surgery, although in the literature we find a wide variability. For example, in a study conducted in England with follow-up of patients treated with colorectal surgery 30 days following hospital discharge,3 a SSI incidence of 27% was recorded; other multicentre study conducted in 140 English hospitals where 6528 colonic procedures4 were assessed, shows a SSI incidence of 10% (95% CI=9.3–10.8); 40.6% of them were organ/space infections.
However, there are studies on colon and rectal surgery that have shown that certain initiatives may have a positive impact for addressing this problem. For example, Hedrick et al.5 implemented a multidisciplinary protocol based on four actions: adequate administration of prophylaxis, to avoid extending prophylaxis for more than 24h, to record and maintain patient normothermia, and to perform perioperative glycaemic control (<200mg/dL). With this protocol, a significant decrease in SSI incidence (from 25.6% to 15.9% [P<.05]) was achieved. Another example is derived from the publication by Forbes et al.6 who conducted a prospective study in two cohorts (pre- and post-implementation of the protocol) of patients who underwent colorectal and hepatobiliary surgery. The protocol consisted in the implementation of Clinical Practice Guidelines (CPG) for the prevention of SSI. Health professionals’ adherence to CPG and SSI rates was assessed and a decrease in SSI incidence from 14.3% to 8.7% (P=.21) was achieved.
In addition to these examples, several publications have shown that surgical procedure monitoring based on feedback by surgeons can significantly decrease infection rates.7–10
The objective of this study is to assess the impact of a SSI improvement programme in colon and rectal surgery on the frequency of surgical infection, its related complications and the mean hospital stay one year and a half after its implementation.
Patients and MethodsStudy PopulationA quasi-experimental study was designed comparing a prospective cohort (after the implementation of the protocol) to a historical cohort. The historical cohort consists of 218 patients, who underwent surgery in two periods (first semester of 2008 and first semester of 2011). The cohort assessed after the implementation of the protocol consists of 124 patients, who underwent surgery in the second semester of 2012.
This study was carried out in the General Surgery Department of Hospital Universitario La Paz (HULP) [La Paz University Hospital] of Madrid, which performs about 3000 interventions in inpatients yearly and is divided into sections per specialty. Patients hospitalised for more than 48h in the General Surgery Department of HULP and treated by major colon and rectal surgery were included. Patients hospitalised for less than 48h were excluded from the study.
A convenience nonprobability sampling was performed and all patients who met the inclusion criteria in the previously defined periods of time were enrolled. The evaluation project was approved by the CEIC (Comité de Ética en Investigación Clínica [Clinical Research Ethics Committee]) of the hospital.
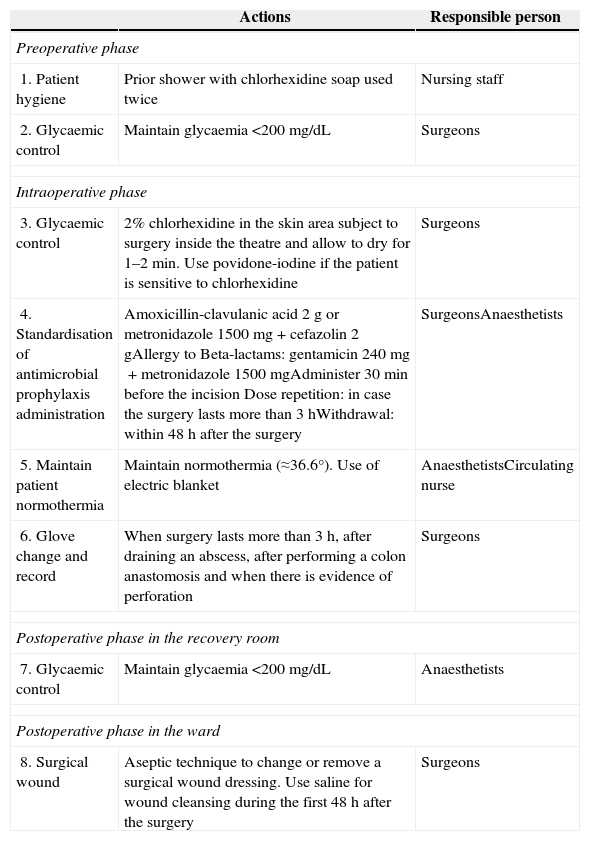
ProtocolThe protocol for the prevention of surgical site infection in colorectal surgery is shown in Table 1. For its implementation, the main clinical practice guidelines were reviewed.
Protocol for Prevention of Surgical Site Infections in Colorectal Surgery.
| Actions | Responsible person | |
|---|---|---|
| Preoperative phase | ||
| 1. Patient hygiene | Prior shower with chlorhexidine soap used twice | Nursing staff |
| 2. Glycaemic control | Maintain glycaemia <200mg/dL | Surgeons |
| Intraoperative phase | ||
| 3. Glycaemic control | 2% chlorhexidine in the skin area subject to surgery inside the theatre and allow to dry for 1–2min. Use povidone-iodine if the patient is sensitive to chlorhexidine | Surgeons |
| 4. Standardisation of antimicrobial prophylaxis administration | Amoxicillin-clavulanic acid 2g or metronidazole 1500mg+cefazolin 2gAllergy to Beta-lactams: gentamicin 240mg+metronidazole 1500mgAdminister 30min before the incision Dose repetition: in case the surgery lasts more than 3hWithdrawal: within 48h after the surgery | SurgeonsAnaesthetists |
| 5. Maintain patient normothermia | Maintain normothermia (≈36.6°). Use of electric blanket | AnaesthetistsCirculating nurse |
| 6. Glove change and record | When surgery lasts more than 3h, after draining an abscess, after performing a colon anastomosis and when there is evidence of perforation | Surgeons |
| Postoperative phase in the recovery room | ||
| 7. Glycaemic control | Maintain glycaemia <200mg/dL | Anaesthetists |
| Postoperative phase in the ward | ||
| 8. Surgical wound | Aseptic technique to change or remove a surgical wound dressing. Use saline for wound cleansing during the first 48h after the surgery | Surgeons |
Antiseptic shower. It is recommended in some guidelines, although some authors consider there is not enough evidence in favour of it compared to a shower with regular soap. In most cases, it is recommended to take a shower with regular soap or with chlorhexidine soap on the same day of the intervention.11
Operative field preparation: current evidence favours a preoperative preparation of the skin with chlorhexidine or chlorhexidine/alcohol solutions such as 2% chlorhexidine in isopropyl alcohol. It is important to allow it to stand (at least 1min) and air dry.12
Antibiotic Prophylaxis- a.
The surgical antimicrobial prophylaxis consists in the administration, preferably single, of at least one antibiotic before the intervention in order to reduce the intraoperative microbial contamination so that it does not overcome the patient's defence mechanisms. The intravenous route is the most frequent route of administration. The antibiotic should be administered between 30 and 60min before incision and at high enough doses to equal or exceed the minimum inhibitory concentration for the microorganism. Such levels should be maintained throughout the intervention and even some hours after it; for that reason, in long surgical procedures, additional doses may be required. In the case of colon and rectal surgery, prophylaxis should be always administered, since it is a surgery which is, at least, contaminated.13
- b.
In some cases, it will be a dirty surgery, that is to say, infected; therefore, antibiotic administration should be scheduled as empirical therapy. For example, this would be the case of a colonic perforation with associated peritonitis.
Colon and rectal surgery requires antibiotic combinations with coverage for the most common microorganisms: anaerobic and gram-negative bacilli. In our protocol, we have taken into account the local and international guidelines.14
Oral antibiotics are not recommended in the current guidelines, since they do not provide better results and may be related to vomiting and abdominal pain.15,16
Preoperative and Postoperative Glycaemic ControlCardiovascular surgery studies showed that maintaining perioperative glucose levels<200mg/dL led to a lower incidence of SSI compared to controls.17,18
However, current CPGs do not recommend a strict glycaemic control as a routine surgical practice due to the risk of hypoglycaemia.11 In our case, it is recommended to maintain blood glucose levels<200mg/dL 24h before and after the surgery.
Glove ChangeGlove change should be performed when the surgery is longer than three hours, after draining an abscess, after performing a colonic anastomosis, and when there is evidence of perforation, according to the measures proposed by observational studies.19–21
Partecke et al.19 showed that wearing gloves for 91–150min resulted in the perforation of at least one of them in 18.1% of studied cases. From 150min onwards, this percentage increased to 23.7%. Out of all the perforated gloves, 66.7% corresponded to the dominant hand, and microperforation was more frequently found in the index finger of that hand. Major abdominal surgeries, vascular and heart surgeries were the ones where more perforations were found.19 However, Misteli et al. showed that surgical glove perforation increases the risk of SSI.21
Maintaining Patient Normothermia in the Operating TheatreHypothermia causes vasoconstriction, decreases tissular oxygen and can reduce the immune response. Several studies have shown a decrease in the SSI incidence in patients subject to normothermia control during the intervention and the postoperative period.22–24
This measure is currently recommended in several CPGs.11,25
Lastly, it should be noted that other actions that were performed in the past, such as mechanical bowel preparation, have been discarded based on the current evidence, since there are studies which show that it does not decrease faecal microorganism concentration in the intestinal lumen and that it can even change solid faeces into liquid ones, which could facilitate bacteria movement towards the wound and towards the peritoneal cavity.26,27
At present, mechanical bowel preparation is contraindicated as a routine measure.28
Implementation of the Protocol and Independent and Outcome VariablesThe protocol was presented in two clinical sessions in the Department of Anaesthesia and the Colorectal Surgery Section. Demographic, clinical, epidemiological and microbiological variables and those related to adherence to protocol were collected. For the diagnosis of infection, CDC criteria were used.29
Statistical AnalysisThe quantitative variables are described as mean, median and standard deviation (SD). Qualitative variables are described with absolute and relative frequencies. Comparisons among quantitative variables were made using the Mann–Whitney test. For comparisons among qualitative variables, the Chi-square test or Fisher's test were used. The analysis was performed with the PASW Statistics 18 programme.
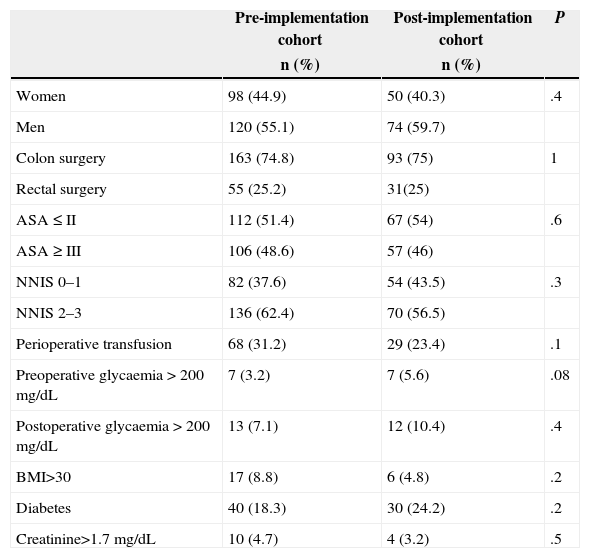
ResultsA total of 342 patients undergoing colon (256) and rectal (86) surgery were assessed; they were divided into two cohorts: a baseline one made up of 218 patients (years 2008–2011) and another one after the implementation of the programme (year 2012) made up of 124 patients. The descriptive study of the main variables in both cohorts and the comparison between them are presented in Table 2. As it can be noted, there are no statistically significant differences between the cohorts.
Bivariate Study for Baseline Comparability of Both Cohorts.
| Pre-implementation cohort | Post-implementation cohort | P | |
|---|---|---|---|
| n (%) | n (%) | ||
| Women | 98 (44.9) | 50 (40.3) | .4 |
| Men | 120 (55.1) | 74 (59.7) | |
| Colon surgery | 163 (74.8) | 93 (75) | 1 |
| Rectal surgery | 55 (25.2) | 31(25) | |
| ASA≤II | 112 (51.4) | 67 (54) | .6 |
| ASA≥III | 106 (48.6) | 57 (46) | |
| NNIS 0–1 | 82 (37.6) | 54 (43.5) | .3 |
| NNIS 2–3 | 136 (62.4) | 70 (56.5) | |
| Perioperative transfusion | 68 (31.2) | 29 (23.4) | .1 |
| Preoperative glycaemia>200mg/dL | 7 (3.2) | 7 (5.6) | .08 |
| Postoperative glycaemia>200mg/dL | 13 (7.1) | 12 (10.4) | .4 |
| BMI>30 | 17 (8.8) | 6 (4.8) | .2 |
| Diabetes | 40 (18.3) | 30 (24.2) | .2 |
| Creatinine>1.7mg/dL | 10 (4.7) | 4 (3.2) | .5 |
| Mean (SD) | Mean (SD) | ||
|---|---|---|---|
| Age | 68.3 (13.1) | 69.7 (13.1) | .3 |
| Surgery time | 172.3 (68.3) | 180.4 (62.8) | .2 |
SD: standard deviation; BMI: body mass index; NNIS: National Nosocomial Infection System.
Cumulative incidences of total SSI and according to its location, for all patients and by procedure are shown in Table 3. The primary outcome, the incidence of total SSI, is significantly lower after protocol implementation. In the case of SSI, however, the reduction was not statistically significant.
Primary Outcome Variables Obtained When Comparing Both Cohorts.
| Pre-implementation cohortNo.=218 | Post-implementation cohortNo.=124 | P | |
|---|---|---|---|
| n (%) | n (%) | ||
| Total SSI | 60/218 (27.5) | 21/124 (16.9) | .03 |
| Type of SSI | |||
| Organ/space SSI | 24/218 (11) | 7/124 (5.6) | .14 |
| Surface SSI | 28/218 (12.8) | 9/124 (7.2) | .15 |
| Deep SSI | 8/218 (3.7) | 5/124 (4) | 1 |
| Procedure | |||
| SSI in colon surgery | 44/163 (26.9) | 15/93 (16.1) | .04 |
| SSI in rectal surgery | 16/55 (29.1) | 6/31 (19.4) | .32 |
| NNIS risk | |||
| SSI NNIS 0 | 1/12 (8.3) | 1/14 (7.1) | 1 |
| SSI NNIS 1 | 10/70 (14.3) | 6/40 (15) | .85 |
| SSI NNIS 2 | 33/99 (33.3) | 7/48 (14.6) | .02 |
| SSI NNIS 3 | 16/37 (43.2) | 7/22 (31.8) | .55 |
| Reintervention | 32/218 (14.7) | 12/124 (9.7) | .2 |
| Death | 20/218 (9.2) | 4/124 (3.2) | .04 |
| Mean (SD) | Mean (SD) | ||
| Days of hospital stay | 18.7 (14.8) | 15.7 (12.4) | .04 |
| Days of postoperative stay | 14.6 (13.08) | 13.3 (11.4) | .3 |
| Days between surgery and SSI diagnosis | 6.3 (3.5) | 8.6 (6.4) | .1 |
SD: standard deviation; SSI: surgical site infection; NNIS: National Nosocomial Infection System;.
Postoperative mortality; (P=.04).
Lastly, the results of the assessment of adherence to protocol are shown in Table 4.
Assessment of Adherence to Protocol.
| Pre-implementation cohortNo.=218 | Post-implementation cohortNo.=124 | P | |
|---|---|---|---|
| n/No. (%) | n/No. (%) | ||
| Antimicrobial prophylaxis | |||
| No record of prescription or administration in the medical record | 15/179 (8.4) | 2/117 (1.7) | .031 |
| No antibiotic withdrawal (a maximum of 48h) | 26/179 (14.5) | 6/117 (5.1) | .018 |
| Antibiotic not included in the protocol and not clinically justified | 4/179 (2.2) | 1/117 (0.8) | .65 |
| Insufficient dose | 20/179 (11.2) | 13/117 (11.1) | .86 |
| Administration delay | 2/179 (1.2) | 0/117 (0) | .52 |
| Compliance with all the antimicrobial prophylaxis requirements of the protocol | 112/179 (62.6) | 95/117 (81.1) | .001 |
| Maintaining normoglycaemia | |||
| Preoperative glycaemic record | 186/218 (85.3) | 115/124 (95) | .007 |
| Postoperative glycaemic record | 184/218 (84.4) | 115/124 (93.8) | .01 |
| Maintaining normothermia | |||
| Use of electric blanket | 51/218 (23.4) | 72/124 (58.1) | .001 |
In our hospital, the implementation of a protocol for the prevention of SSI has yielded a decrease from 27.5% to 16.9% in the total frequency of these infections; this decrease is similar to that described by other authors.5,30–33
For example, Hendrick et al.5 achieved a SSI decrease in colorectal surgery from 25.6% to 15.9% (P<.05) after the implementation of a multidisciplinary protocol, with an increase in the percentage of adequate administration of prophylaxis from 68% to 91% (P<.001), among other measures. The study conducted by Wick et al.32 also shows a 33.3% decrease in SSI in colorectal surgery 12 months after the implementation of a programme based on preoperative patient hygiene with chlorhexidine, standardisation of surgical skin preparation, maintainence of patient normothermia and the adequate administration of antibiotic prophylaxis.
In our case, protocol measures were also implemented in a care bundle fashion, that is to say, simple measures, which must be performed together, and which are thus potentiated. One of the measures was the protocolisation of antibiotic prophylaxis, which has shown to be effective in surgical processes since long ago and it is, therefore, a measure applied in hospitals in our region. Nevertheless, it does not fit in with all parameters (type of antimicrobial drug, administration time and withdrawal time) uniformly in all the centres, and even in the same hospital there may be differences among departments or among professionals. We considered that a non-justified variability was not acceptable and were very strict when considering prophylaxis as “inadequate” because it did not meet all the proposed parameters of the protocol.
In addition to the positive results on infection, our study shows a significant decrease in postoperative mortality. It is not strange that both results have gone together, since previous publications have already shown this19,31: we highlight the publication of Crolla et al.,31 which shows a similar decrease to that of our study in SSI, mean hospital stay and mortality after the implementation of a bundle. For our part, as shown in the Results section, we have conducted a study on risk factors for postoperative mortality in all studied patients and both SSI of any type and organ/space SSI proved to be independent risk factors. For hospital stay, in our study, a decrease was also observed in the post-implementation group with a mean of three days.
Adherence to protocol has been evaluated using a series of variables related to compliance with the main measures. As presented in Table 4, most items show a significant improvement. Notwithstanding, all measures could not be evaluated; for example, temperature could not be recorded during the intervention, although the use of an electric blanket was satisfactorily recorded. We could not record glove change either; therefore, we do not know adherence to this measure and cannot quantify its impact on final outcomes.
Lastly, regarding adherence to protocol, it should be noted that the main advantage we have had is that measures did not require any costs and most of them had already been partially introduced; therefore, its systematisation has not involved any kind of additional reorganisation.
In order to conclude the discussion on results, we should mention that in some studies the authors have not observed improvements in SSI rates after the implementation of similar measures. In relation to data from our region, the publication on a multicentre study conducted in 19 Catalonian hospitals stands out. The authors recorded a SSI incidence in colon cancer surgery of 23.2% (95% CI: 18.9–27.6), which did not vary after the application of a bundle of preventive measures.34
Considering study limitations, we should highlight that, due to the outcome assessment design, which compares patients who underwent surgery some years ago, we cannot state that these results are due to the protocol alone, since there may be other uncontrolled factors that could have contributed to this. For example, each surgeon's experience or the technical improvements that may have taken place over the years are difficult-to-control factors. Other factors that may have had an influence on the results include the further knowledge on SSI-related factors acquired over time or, in our case, the specialisation since the General Surgery Department was reorganised in sections.
It should also be noted that, although the control or historical cohort was prospectively collected, some variables were retrospectively collected for this study. This implies that some data could not be collected in all patients, for example, patient's height and weight for BMI calculation.
Another limitation that should be taken into account is the “observational bias”, given that after protocol implementation and clinical sessions offered in the departments, health professionals knew that they were highly likely to be audited, since we had committed to provide feedback on results at least biannually.
Nevertheless, and in spite of the abovementioned methodological limitations, we believe that the implementation of evidence-based, simple, feasible recommendations together with a strong leadership from the responsible clinicians involved, are useful tools to improve surgical patient safety, as shown at a local level. We base such statement on the previously mentioned analysis, where a significant decrease in the frequency of SSI, postoperative mortality and inadequate antimicrobial prophylaxis is verified.
Conflicts of InterestThe authors declare that they do not have any conflicts of interest.
Please cite this article as: Pérez-Blanco V, García-Olmo D, Maseda-Garrido E, Nájera-Santos MC, García-Caballero J. Evaluación de un paquete de medidas para la prevención de la infección de localización quirúrgica en cirugía colorrectal. Cir Esp. 2015;93:222–228.