Gastrointestinal stromal tumors (GIST) are the most frequent mesenchymal tumors in the digestive tract. Most have a characteristic gain-of-function mutation of the c-KIT gene, which encodes the KIT receptor (CD117).1 The availability of an inhibitor for said receptor, imatinib mesylate, plays an important role in the chemotherapy treatment of high-risk, metastatic and/or unresectable GIST.2–4 For the anatomic pathology diagnosis of GIST, immunohistochemical staining is used. 95% express CD117 and DOG1, in addition to others markers such as CD34 (60%–70%), smooth muscle actin (15%–60%), S100 protein (10%) and desmin (rarely).5 However, although the expression of CD117 in the context of a high degree of suspicion is generally diagnostic of GIST, there are other tumors that are morphologically similar and express CD117, including Ewing's sarcoma.6,7
We present the case of a 35-year-old male, with no medical history of interest, who was admitted for further study of edema of the lower right leg and a palpable abdominal mass. A computed tomography scan showed evidence of a large pelvic mass measuring 20×13×18cm, suspected of being a mesenchymal tumor. Percutaneous biopsy showed a mesenchymal proliferation of epithelioid-type cells with mild nuclear pleomorphism and small nucleoli, arranged in a diffuse pattern. Areas of necrosis occupied 50% of the volume of the biopsied tumor tissue. The immunohistochemical study demonstrated expression of CD117 (KIT) by the neoplastic cells and negativity for DOG1, CD34, smooth muscle actin, desmin, S100 protein, cytokeratins and EMA. Given the morphology, immunohistochemical expression of KIT and intra-abdominal location, the mass was identified as an epithelioid GIST and neoadjuvant treatment with imatinib was initiated.
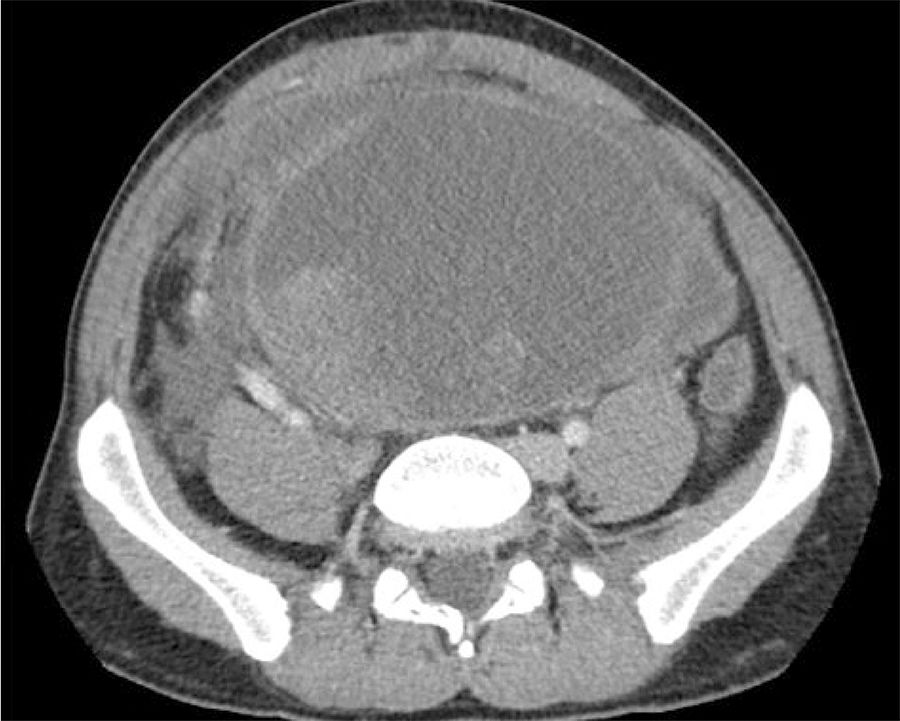
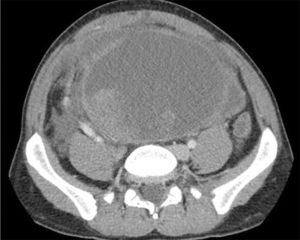
After one month of treatment and no clinical response, the patient came to the emergency room with intense and continuous pain in the right iliac fossa and persistent constipation. Upon examination, a large palpable abdominal mass was detected, along with distension, pain and signs of peritoneal irritation. Computed tomography demonstrated the large solid cystic mass with no significant changes. Tumor bleeding could not be ruled out and free fluid was observed, in addition to right grade II hydronephrosis due to ureteral entrapment (Fig. 1) and secondary decline in renal function.
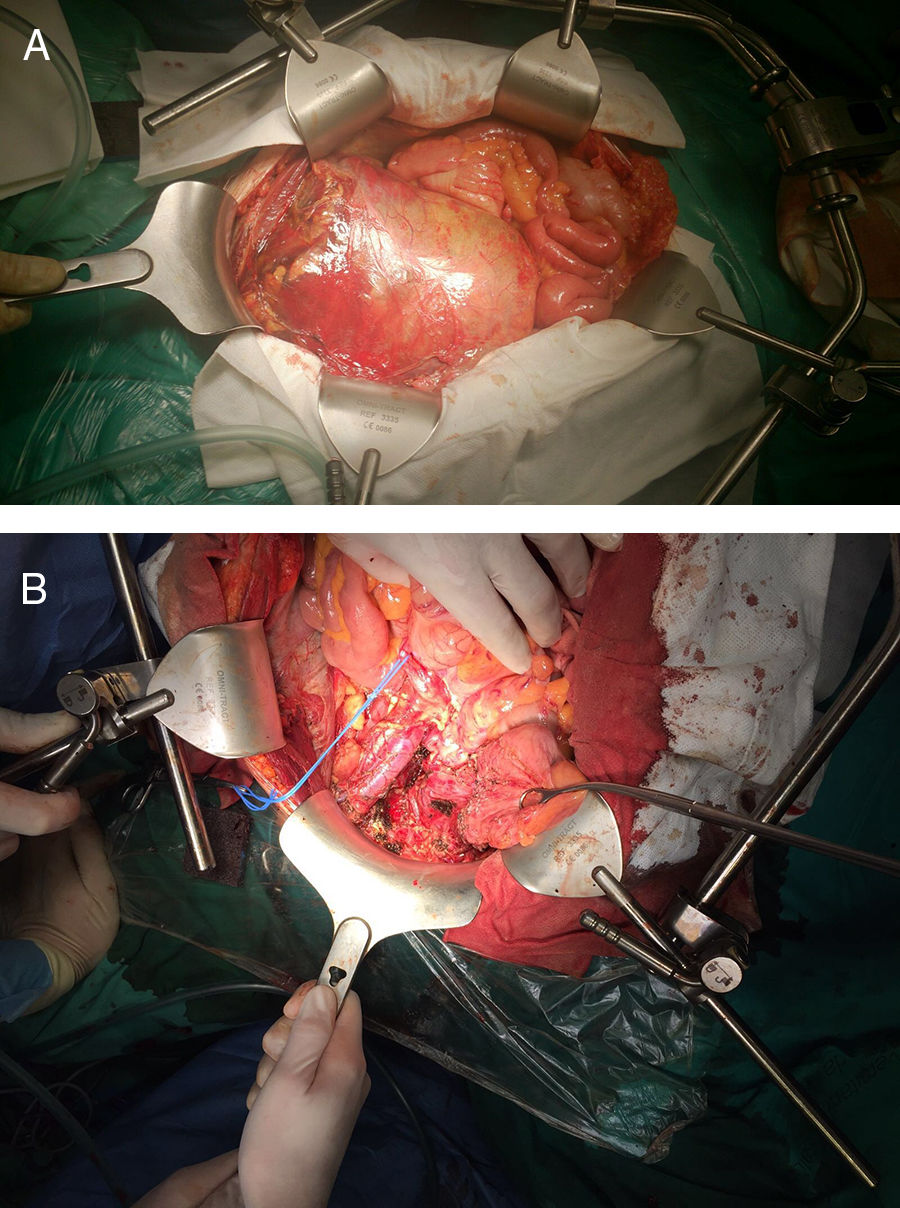
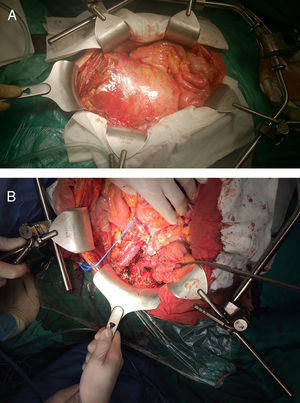
Urgent surgery was performed, which revealed a preperitoneal mass adhered to the right external iliac artery and vein as well as the ureter, displacing the bladder to the left iliac fossa (Fig. 2A). The tumor was resected, dissecting to the right obturator orifice and right iliac spine, with partial division of the pectineus muscle and opening of the tumor in that region. The margin was subsequently extended to the periosteum (Fig. 2B). The patient was discharged on the eighth postoperative day. His only complication was postoperative right obturator neuropathy (Clavien II). The histopathological study identified areas of epithelioid cells found in the biopsy as well as minor areas of small round cells, foci of clear cells and cells with rhabdoid morphology. The immunohistochemical study was extended, which, together with the KIT expression, showed a strong and diffuse membranous positivity for CD99. Fluorescence in situ hybridization was done with a break-apart probe, which showed evidence of EWSR1 translocation; RT-PCR amplification and Sanger sequencing detected EWSR1 fusion (exon 7)–FLI1 (exon 6). The PCR amplification study of exons 9, 11, 13 and 17 of the C-KIT gene and of exons 12, 14 and 18 of the PDGFRA gene with Sanger sequencing did not detect mutations in these genes. Based on the histopathology, immunophenotype and molecular findings, the final pathological diagnosis was atypical Ewing's sarcoma (pT4 L0 V0 R1). Cytology of the ascitic fluid was negative for malignant cells.
The patient participated in a clinical trial and completed 15 cycles of chemotherapy, with no evidence of recurrence 15 months after the intervention.
One of the accepted theories about the origin of the Ewing sarcoma family of tumors (ESFT) is that they arise from mesenchymal stem cells present in the organism and are capable of transforming into different types of tissue. ESFT are undifferentiated neoplasms of varying degrees with multiple phenotypic expression, all sharing a gene alteration in the EWSR1 gene (chromosome 22q12) and positive immunohistochemical staining for CD99 in most cases. In 65% of cases, CD117 positivity is also present, similar to GIST.8,9
With an incidence of 3 cases per million people, ESFT are very rare, and 90% occur between the ages of 5 and 25. ESFT include extraskeletal Ewing's sarcoma, although it is less frequent than the bone type, with a prevalence of 15%–20% of all Ewing's sarcomas. Its most frequent location in the paravertebral region (32%) and the lower limbs (26%), and less frequently the chest wall (18%), retroperitoneum (11%), as in our case, pelvis (11%) and the upper limbs (3%). Its presentation is as a soft tissue mass with no bone marrow involvement on magnetic resonance imaging studies.10
The tumor histopathology, location, immunohistochemical and molecular studies are essential for making a correct diagnosis. However, the clinical case and imaging tests also play a fundamental role in guiding the diagnosis.
In the case presented, the epithelioid morphology of the cells, the immunohistochemical expression of CD117 and the patient being a 35-year-old man with an asymptomatic intra-abdominal mass guided the diagnosis toward GIST, which led to the initiation of treatment with imatinib to reduce the mass and be able to propose R0 surgical resection. The lack of clinical response and the intraoperative finding of adhesion to the right ischiopubic ramus were explained after the definitive diagnosis of atypical Ewing's sarcoma.
In conclusion, for future cases it would be appropriate to include atypical intra-abdominal Ewing's sarcoma in the differential diagnosis of GIST in order to indicate the correct treatment from the start.
Please cite this article as: Rihuete C, Acín-Gándara D, Pereira F, Tardío JC. Sarcoma de Ewing atípico: un diagnóstico diferencial a tener en cuenta en los tumores del estroma gastrointestinal (GIST). Cir Esp. 2019;97:112–114.
This case report was presented as a poster at the 21st National Surgery Congress held in Malaga, Spain, October 18–20, 2017.