Enteric duplication cysts are a congenital entity without familial predisposition, whose incidence is estimated at one per 4500 births; they can be located at any level of the gastrointestinal tract.1 Gastric duplication cysts (GDC) were first described by Wendel in 1911. Their prevalence is 2%–8% of all enteric duplication cysts, making them the second rarest type only after appendiceal duplication cysts.2
Their etiology is brought about by alterations in embryonic development, although there is no theory that fully explains their formation. Their morphology may be tubular, or more frequently cylindrical. GDC originate dorsally to the primitive intestine, which is why most of them are located in the greater curvature, generally distal, and only 5.5% are located in the lesser curvature.
They are more frequent in women and usually diagnosed during childhood by the symptoms they cause, while in adults their diagnosis is usually incidental. In more than 50% of cases, they are usually associated with other malformations, such as esophageal duplications or vertebral alterations.3
The aim of this article is to describe a case of GDC as an incidental finding in an adult, which presented rare characteristics and location. This case demonstrates the difficult preoperative diagnosis of this pathology, mainly in its differential diagnosis with gastrointestinal stromal tumors (GIST).
The patient is a 45-year-old man with no medical history of interest. He was referred to us due to the incidental finding by computed tomography of a 35-mm hypodense nodular lesion in the region of the gastrohepatic ligament, adjacent to the lower gastric curvature, with peripheral linear calcification. Initially, these findings were compatible with an enteric duplication cyst (Fig. 1). Clinically, the patient only reported occasional sharp epigastric discomfort.
An esophagogastroscopy was performed, which only provided the finding of inactive chronic antral gastritis. Two endoscopic ultrasound studies were conducted. The first revealed a hypoechoic lesion measuring 29×24mm at the subcardial level that was dependent on the muscularis propria, with a central anechoic area suggestive of necrosis and a peripheral line of calcification occupying less than 50% of the circumference. No perilesional lymphadenopathies were observed. The lesion, which was subepithelial, was suggestive of GIST. Given the disagreement of the previous tests, we decided to repeat the endoscopic ultrasound, which was suggestive of GIST, and the study was completed with fine-needle aspiration (22G, 3 passes). The biopsy sample was reportedly blood and mucus, with some inflammatory elements and no histological signs of malignancy. Finally, a gastroduodenal barium study only demonstrated a small mass effect at the esophagogastric junction. Serum levels of Ca19.9, CEA and Ca 125 were within normal ranges.
Given the suspicion of a GIST and despite not having histological confirmation, during the case assessment by the Digestive Tumors Committee it was decided to perform laparotomy. Intraoperatively, a gastric tumor was found adjacent to the esophagogastric junction, where partial or atypical resection could not be done. Therefore, total gastrectomy was conducted with a Roux-en-Y esophagojejunostomy. The postoperative period was uneventful, and the patient was discharged on the 9th postoperative day.
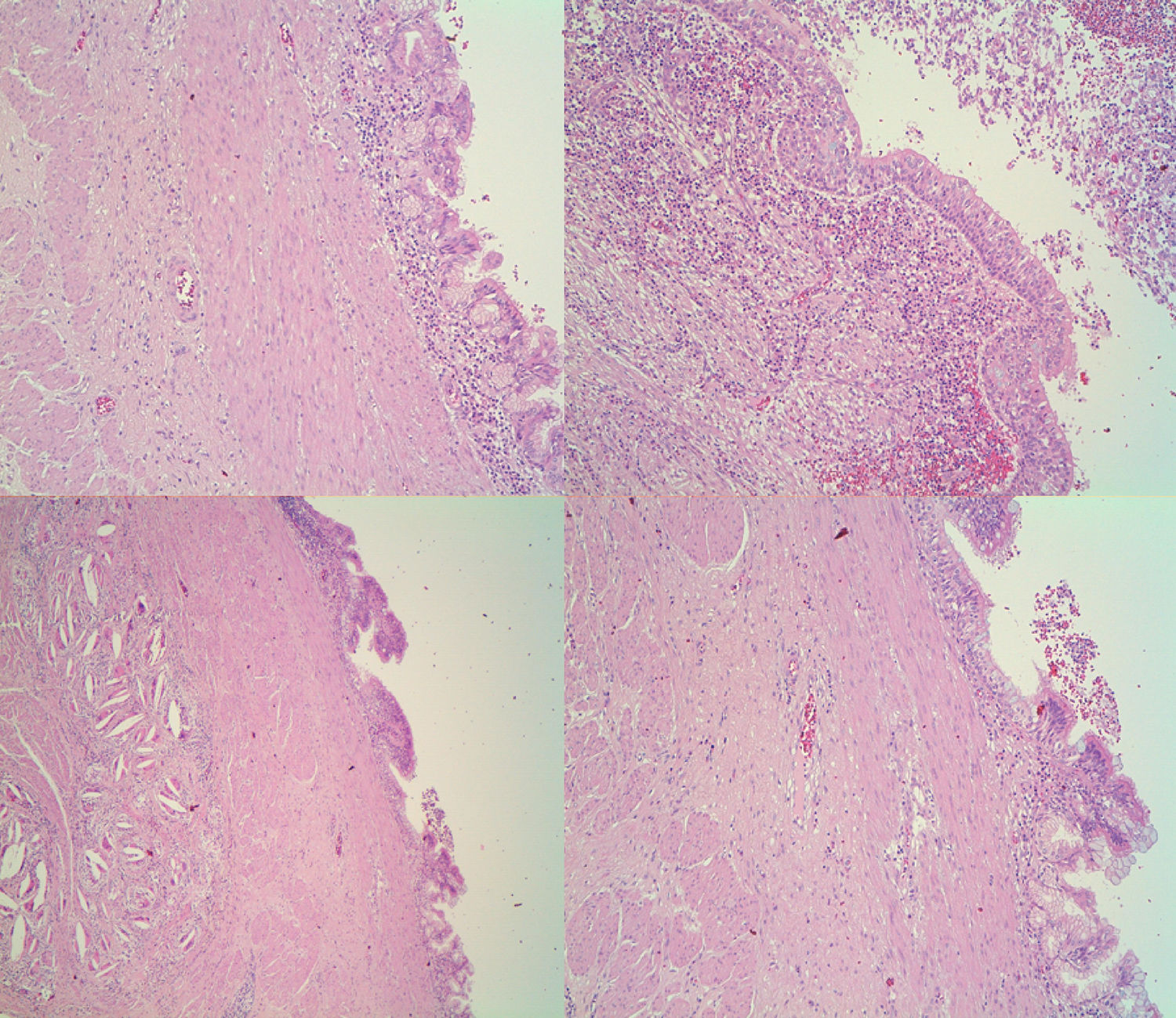
In the histology study of the surgical specimen, a lesion was observed macroscopically in the lesser curvature at a distance of 1cm from the esophagogastric junction that was submucosal, measuring less than 2.6×2.5×2.5cm and unilocular cystic with a partially calcified wall. The histological sections showed that the cyst wall had a respiratory epithelial lining with partial calcification and transitional mucosa of the esophagogastric junction in continuity with the mass, but no signs of malignancy (Fig. 2). The definitive diagnosis was a GDC, with no evidence of malignancy in the 17 isolated lymph nodes.
Abdominal computed tomography and MRI are able to identify GDC, but diagnostic errors are frequent in solid lesions. Most authors report that endoscopic ultrasound offers greater diagnostic efficacy for these tumors, while allowing for needle aspiration to obtain histological material, which is essential for the differential diagnosis and to rule out malignancy.4 In our case, the 2 endoscopic ultrasounds performed suggested an incorrect diagnosis.
The definitive diagnosis requires histological confirmation according to Rowling's criteria: proximity to the digestive tract, common blood supply, smooth muscle layer shared with the gastrointestinal wall and digestive epithelium lining.5 However, cases have been described with heterotopic mucosa, such as pancreatic mucosa and respiratory epithelium, as in the case we present.
The differential diagnosis should include cystic lesions and GIST, and the definitive diagnosis is reached by excision of a surgical piece.6
At present, there is no consensus regarding treatment. In symptomatic cases, the recommendation of surgery is undisputed. In asymptomatic cases, there are authors who also advocate surgical excision due to the risk of malignization (adenocarcinomas,7 squamous cell carcinoma and neuroendocrine tumor with different degrees of malignancy) or complications (superinfection, hemorrhage, fistula, etc.), although these are infrequent.8
In mediastinal cysts, Zambudio et al. propose criteria for surgical excision (symptomatic, progressive growth, infection, atypical characteristics, etc.) that could be extrapolated to GDC.9
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: García Nebreda M, Paseiro Crespo G, Álvaro Cifuentes E, Marqués Medina E, Burdaspal Moratilla A. Quiste de duplicación gástrica de epitelio respiratorio: una lesión infrecuente de difícil diagnóstico diferencial. Cir Esp. 2019;97:57–59.