Enteral nutrition is an option in patients for whom oral nutrition is not possible or sufficient. When required for prolonged periods, percutaneous endoscopic gastrostomy (PEG) is a good alternative, as it presents fewer complications, with greater comfort and tolerability, than nasogastric or nasojejunal tubes.1 Complications associated with PEG include pneumoperitoneum, gas in the portal or mesenteric vein, transverse colon injury, gastrocolic fistula, small bowel lesion, hepatic or splenic injury, and peritoneal or abdominal wall bleeding. These complications are uncommon. In most cases, they are detected during the completion of the gastrostomy and resolved during the same procedure. In long-term gastrostomies, after peristaltic manipulation, possible complications include: peristomal pain, abdominal wall abscess (18%), necrotizing fasciitis, peristomal herniation or fistula (1%–2%), gastric bleeding and decubitus ulcer (2.5%), gastric obstruction, paralytic ileus or gastroparesis, gastric volvulus or pulmonary aspiration (<1%).1
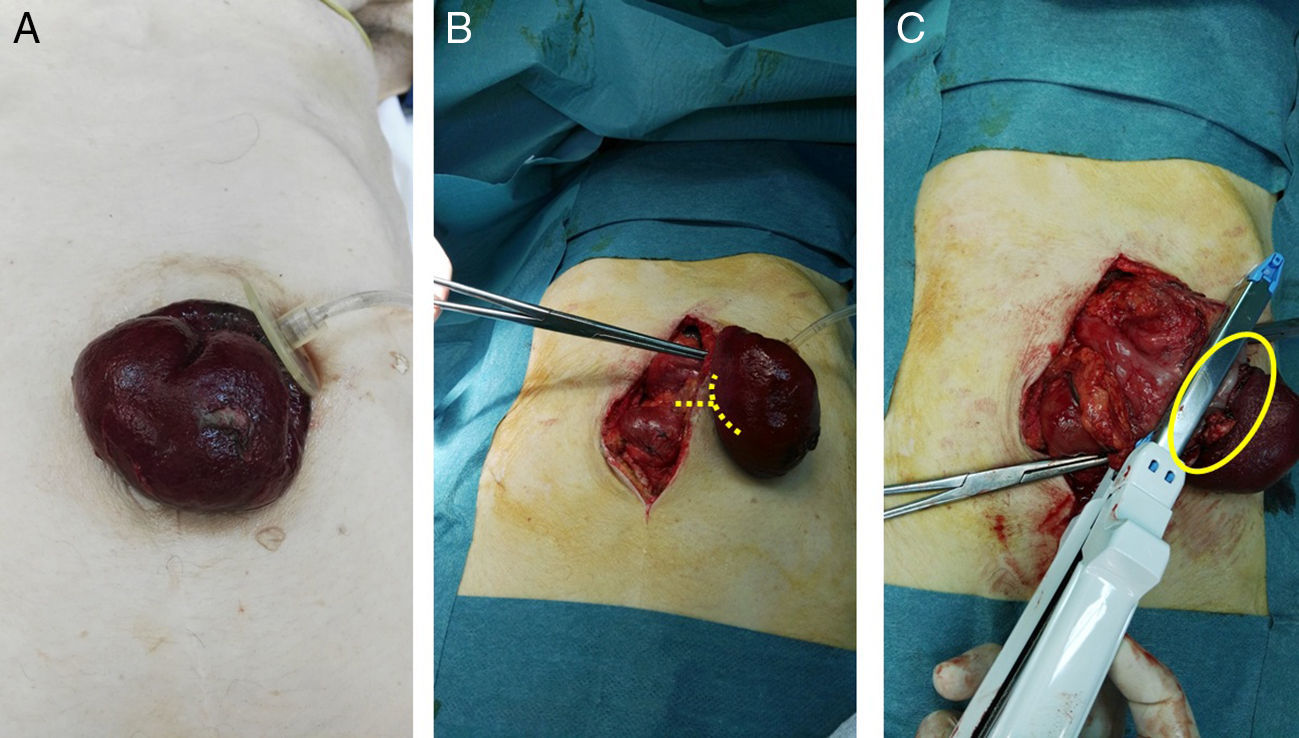
We present the case of a 75-year-old woman with permanent percutaneous gastrostomy due to a cerebrovascular accident associated with oropharyngeal dysphagia 3 years earlier, who came to the emergency room due to abdominal pain accompanied by nausea and gastrostomy prolapse for approximately 12h with signs of acute ischemia and necrosis of the gastric wall. Upon physical examination, she presented a BMI of 18kg/m2 with a decline in her general condition, tachycardia and hypotension. Examination showed a soft abdomen that was painful to deep palpation, but no signs of peritoneal irritation. Gastric prolapse was observed through the gastrostomy orifice with signs of ischemia in the gastric mucosa (Fig. 1A). Lab work showed discrete leukocytosis of 14900, with 88.1% neutrophils.
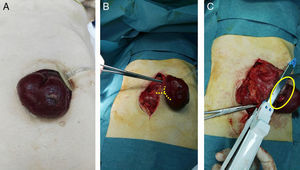
(A) Gastric prolapse through the gastrostomy orifice with signs of gastric mucosal suffering; (B) mean laparotomy and release of the gastrostomy ring; the lines point to the dissection point of the neck of the stomach strangulation; (C) necrosis of the total thickness of the gastric wall and partial gastric resection using the GIA; the circle shows the dissected neck.
With the diagnosis of gastric prolapse strangulated through the orifice of the gastrostomy, urgent surgery was indicated. A midline laparotomy was performed, and the gastrostomy ring was freed (Fig. 1B), which was not dilated; ischemia was observed with necrosis of the entire thickness of the gastric wall, so partial gastric resection was performed with a reloadable linear cutter (GIA) during open surgery (Fig. 1C) with invagination of the staple line using 2/0 silk. To do this, the gastrostomy balloon was deflated, which had remained inflated and in the abdominal cavity below the hernial ring. Subsequently, given the history of oropharyngeal dysphagia, a Stamm neogastrostomy was carried out using a Pezzer catheter in the gastric fundus with a double purse-string and fixation with nonabsorbable suture to the parietal peritoneum. Once the Pezzer was affixed and before closure of the laparotomy, we checked for leaks with the passage of saline solution to the duodenum, which ruled out distal obstruction as a cause of the prolapse. During the postoperative period, management was based on bowel rest and total parenteral nutrition. Nutrition was initiated by gastrostomy on the third day post-op, and the patient was discharged 7 days after hospitalization.
This case is exceptional because of its presentation as gastric prolapse strangulated through the orifice of a gastrostomy, possibly triggered by low BMI, malnutrition and weakness of the abdominal wall tissue. The patient required urgent surgery. There are few reports in the literature with similar characteristics.2,3
A prolapsed stoma of the small intestine or colon through the abdominal wall is relatively common. However, there have only been isolated reports of gastric prolapse through the gastrostomy orifice, which is uncommon.4,5
The concept of gastrostomy prolapse is ambiguous since it includes both the migration of the gastrostomy catheter from the stomach toward the duodenum or the esophagus as well as the exteriorization of part of the stomach through the gastrostomy port outside the abdominal cavity. Migration of the gastrostomy catheter is relatively frequent, with an incidence of between 0.4% and 11%. Its resolution is simple and involves pulling the catheter for correct relocation. In our case, there was a gastrostomy prolapse with exteriorization of the gastric wall through the orifice of the gastrostomy. Several primary conditions have been described in association with gastric prolapse through the gastrostomy orifice: cerebral palsy, dementia, cerebrovascular accident, Parkinson's disease, cloacal atresia, congenital short bowel syndrome, Cornelia-Lange syndrome, diaphragmatic hernia, severe gastroesophageal reflux, necrotizing enterocolitis, progeria, pulmonary hypoplasia, amyotrophic lateral sclerosis, tumors of the upper gastrointestinal tract, tracheoesophageal fistula, etc. In addition, other authors6 have proposed as possible causes the presence of catheter-related fistulae with gastric content associated with cutaneous excoriation and injury.7
Mitchell and Tetroe8 pointed out some factors that may influence the occurrence of complications following the creation of a PEG, such as advanced age, presence of malignant disease, male gender and hypoalbuminemia. Another study by Blomberg et al.9 shows how 30-day mortality after PEG increases in patients with low albumin values, elevated C-reactive protein, age over 65 and BMI below 18.5m/kg.2,10,11
In any case, the incidence found in the literature of prolapse of the entire thickness of the gastric wall through the gastrostomy orifice described in our case report is uncommon and requires, in most cases, a laparotomy for its correct resolution.
Please cite this article as: Fernández-SanMillán D, López-Tomassetti Fernández E, Hernández Hernández JR. Necrosis gástrica secundaria a prolapso de gastrostomía percutánea. Cir Esp. 2017;95:474–475.