The pancreaticoduodenal groove is a space outlined by the pancreas, duodenum and common bile duct. Pancreatitis is one of the diseases that can affect this anatomical area. It was described for the first time in 1973 by Becker and Bauchspeinchel1 and, in 1982, Stolte et al. coined the term groove pancreatitis. It is a rare entity characterized by chronic segmental2 pancreatitis and has an uncertain pathogenesis.3 Its identification is important due to the diagnostic problems that may arise with other serious diseases that affect the head of the pancreas, such as pancreatic cancer.
We present the case of a 42-year-old woman with a history of thrombophilia and a heavy drinking habit who, in August 2011, complained of continuous epigastric abdominal pain radiating to both hypochondria during the previous 5 months. The pain had worsened in the last week and was accompanied by postprandial heaviness, vomiting, intermittent 38°C fever and weight loss. On examination, the patient showed only mild fever and epigastric tenderness.
The lab work only showed alterations in hemoglobin (11.3g/dl) and leukocyte (15.000/μl) levels, with elevated PCR. Tumor markers CEA and CA 19.9 were normal.
Abdominal computed tomography (CT) revealed a mass measuring 50mm×65mm in the head of the pancreas/uncinate process that seemed to encompass the duodenum, along with increased density of the perilesional fat and retroperitoneal lymphadenopathies. Upper gastrointestinal endoscopy showed no lesions. Endoscopic ultrasound, however, revealed extrinsic compression in the second part of the duodenum with normal mucous membranes and a heterogeneous echogenic mass with irregular edges in the head of the pancreas/uncinate process. Fine-needle aspiration reported an inflammatory process. Magnetic resonance imaging (MRI) confirmed the CT findings. The possibility of an autoimmune process was ruled out, as antibodies (antinuclear, anti-lactoferrin, anti-neutrophil cytoplasmic PR3 and MP0) and immunoglobulin (IgG, IgG4, IgA and IgM) were within normal ranges.
The patient was symptomatic after hospital discharge and, given the suspicion of an inflammatory process, and was monitored with periodical follow-up analyses and radiological studies, which continued to indicate an inflammatory process.
One year after hospitalization, the patient once again presented similar symptoms, with minimal elevation of serum amylase (203U/l). CT confirmed the previous findings, and the patient was discharged after improvement in the symptoms.
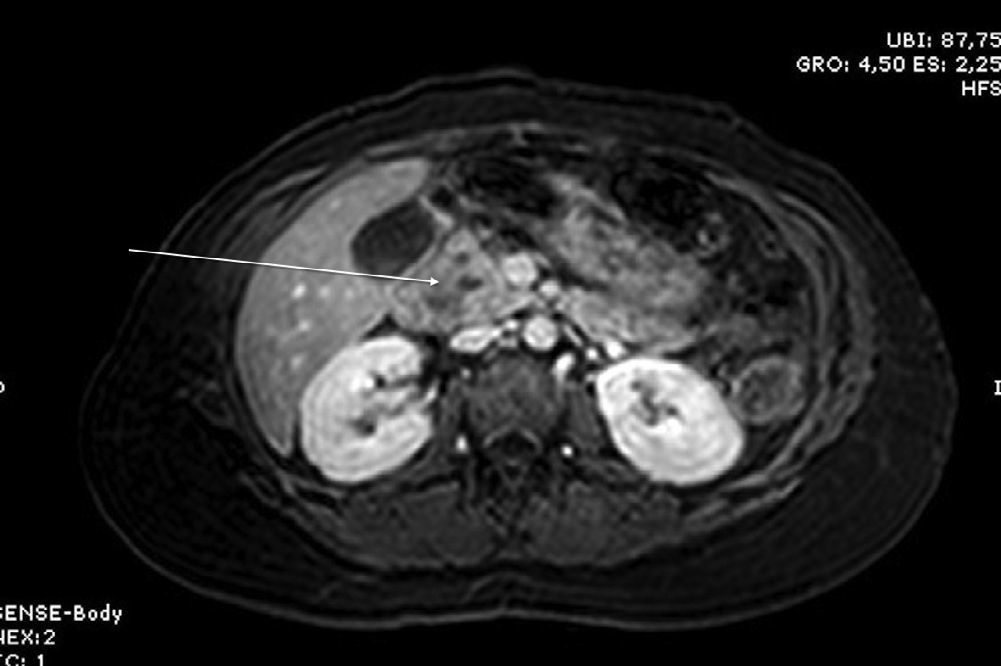
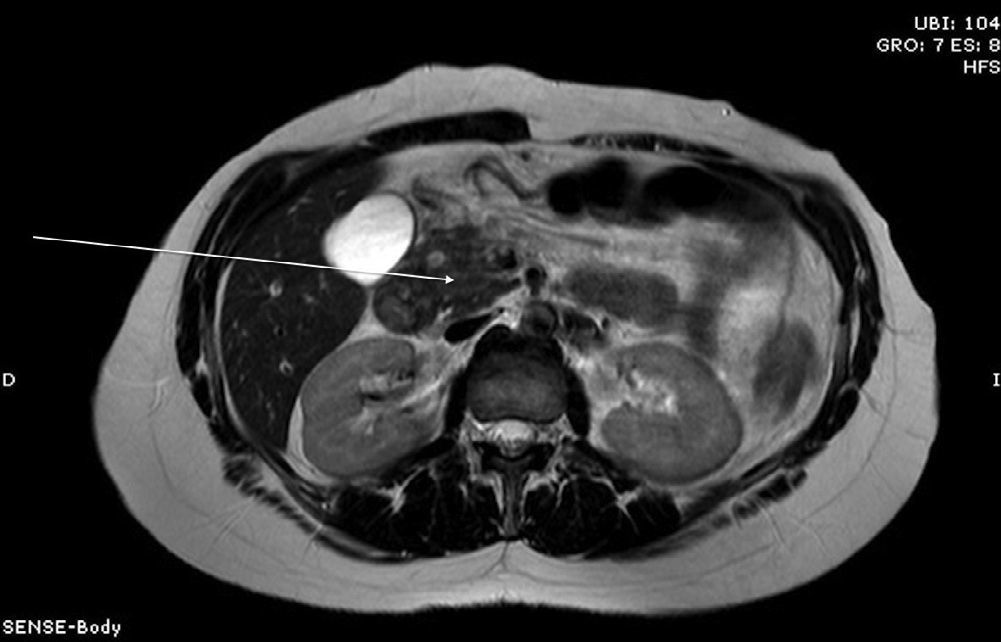
Three months later, an MRI study showed a solid-cystic heterogenous mass measuring 7.6mm between the head of the pancreas and the duodenum; it was hypointense in sequence T1 and hyperintense in sequence T2, with no contrast uptake. Residual inflammatory changes were also observed in the pancreaticoduodenal space as well as wall thickening of the second part of the duodenum, consistent with focal groove pancreatitis (Figs. 1 and 2).
With the diagnosis of groove pancreatitis, symptomatic treatment and abstinence from alcohol were continued, and the patient remains asymptomatic to date.
Groove pancreatitis is a relatively unknown presentation of chronic pancreatitis, which consists of the appearance of fibrous-scar tissue in the fatty plane of the pancreaticoduodenal groove. It most frequently affects middle-aged men with a history of alcoholism.4 Two types have been described (pure and segmental) according to whether only the groove is affected or whether the dorso-cranial part of the head of the pancreas is affected as well.5
Symptoms include postprandial abdominal pain, vomiting, weight loss and, less frequently, jaundice.4
The pathogenesis is uncertain, and different causes have been proposed: peptic ulcer, gastric resection, duodenal wall cysts, presence of heterotopic pancreas in the duodenal wall and anatomical variations in the region of the minor papilla, associated with high alcohol consumption, which would increase the density of the pancreatic fluid and its proteins.3
Diagnosis is based on clinical suspicion and different diagnostic tests. For many authors, endoscopic ultrasound is the diagnostic test of choice as it has greater sensitivity (86%) and specificity than conventional abdominal ultrasound and biopsies can be taken.6 Gastroesophageal studies and upper gastrointestinal endoscopy are also useful as they are able to identify duodenal stenosis, and ERCP can display mild stenosis of the main pancreatic duct. CT usually identifies a mass with laminar morphology between the head of the pancreas and the second part of the duodenum that is hypodense with enhancement after administering contrast, although the findings are not completely specific. MRI generally locates a laminar mass in the pancreaticoduodenal groove that is hypointense compared with the pancreatic parenchyma in T1 and isointense or mildly hyperintense in T2, with delayed enhancement after administering gadolinium,6 as occurred in our case.
According to Gabata et al.,7 the differential diagnosis between groove pancreatitis and adenocarcinoma cannot be done exclusively with CT and MRI studies. This is especially true if there are no cysts within the mass or in the thickened walls of the duodenum, requiring duodenal biopsy or arteriography. In our case, the diagnosis was based on clinical suspicion after a biopsy taken with endoscopic ultrasound that suggested an inflammatory process. MRI images later confirmed the groove pancreatitis.
In pure forms, the differential diagnosis should be made with cholangiocarcinoma and acute pancreatitis with abscess in the area of the groove. The segmental form requires differential diagnosis with pancreatic adenocarcinoma, which is difficult but of great importance; in some cases, the differential diagnosis is only achieved after pancreaticoduodenectomy.8
Conservative therapy based on analgesics, pancreatic rest and abstinence from alcohol are the mainstays of groove pancreatitis treatment. These measures usually succeed initially and must be regularly re-evaluated according to the symptoms, imaging studies and lab determinations.3,6,8,9 On occasion, the symptoms are resistant to medical treatment and surgical intervention may be required. Pancreaticoduodenectomy is the procedure of choice,9 and there are case reports of resection of the head of the pancreas with duodenal preservation10 or even bypass in high-risk patients.10 Surgery may be necessary if it is not possible to definitively rule out pancreatic cancer.
Please cite this article as: Palomeque Jiménez A, Pérez Cabrera B, Navarro Freire F, Jiménez Ríos JA. Pancreatitis del surco en el diagnóstico diferencial del adenocarcinoma de páncreas. Cir Esp. 2014;92:127–129.