Inflammatory pseudotumor of the liver (IPL) is a very uncommon pathology. Its etiology and pathogenesis have not been clarified, and correct preoperative diagnosis is not frequent.1,2 We present the case of a patient who had undergone surgery for breast cancer; during follow-up, PET-CT showed a false positive metastatic lesion, whose final histologic diagnosis was IPL.
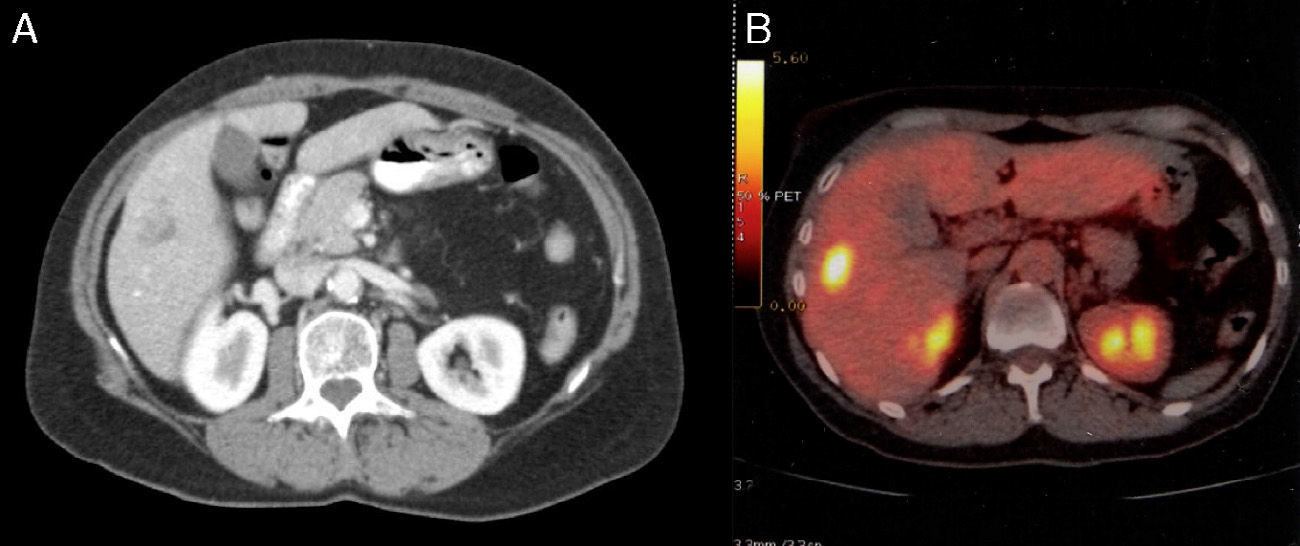
Case ReportThe patient is a 55-year-old woman with a history of invasive ductal carcinoma of the breast (pT2, pN1b, cM0, G3) and negative hormone markers in July 2012, who had been treated with Taxotere® and bevacizumab. In Abril 2013, a CT scan detected a poorly defined, hypodense lesion measuring 14mm in segment VI with 2 small adjacent punctiform calcifications (Fig. 1A). On PET-CT scan, the lesion measured 18×14mm and SUV was 5.9 (Fig. 1B). We performed atypical segmentectomy of the lesion located between segments V and VI. The patient had no complications and was discharged on the fourth day. On the PET study done 6 months later, there was no further tumor activity in the liver.
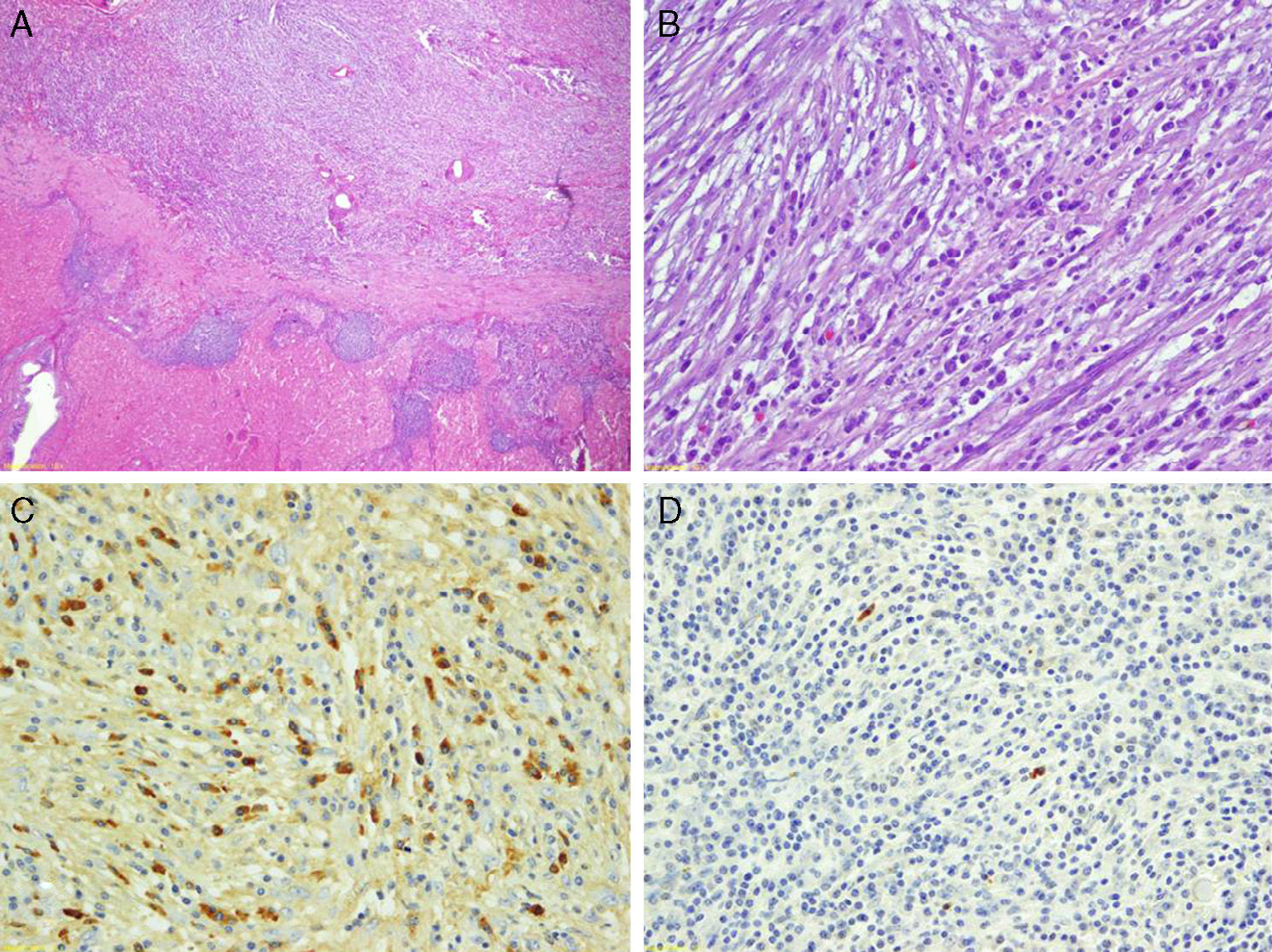
The macroscopic study of the surgical specimen showed it to weigh 60g and measure 6cm×4cm×4cm. It presented a well-defined, rounded, yellow nodule that was not encapsulated measuring 1.5cm, which was not in contact with the surgical margins. Histopathologically, it was made up of a proliferation of spindle cells with an abundant inflammatory component (Fig. 2). The mesenchymal cells showed a certain storiform pattern and were interspersed with areas of edema. The inflammatory infiltrate was practically exclusively plasma cells, which were predominant in the periphery of the lesion. The thin vessels were observed to have inflammation, occasional images of obliterative phlebitis and hemosiderin residue. Immunohistochemistry was not positive for pan-keratin (AE1/AE3), keratin 7, estrogen and progesterone receptors, CD34, alpha-actin, actin HHF-35 or desmin. The ki-67 proliferation rate was 2%. IgG was positive in an important number of plasma cells, but only 2% were IgG4 (3 per high-power field). Serum IgG4 determination was normal.
DiscussionInflammatory pseudotumors are a heterogenous group of solid lesions located in different organs, characterized by an inflammatory infiltrate as the predominating cell component.3,4 The liver is the second most common organ where they appear.2
IPL are uncommon lesions. There are only 300 cases published in the medical literature, the first of which was reported by Baker in 1953. They are benign, usually solitary tumors, located in the right liver, that can be confused with other benign and malignant hepatic lesions (liver abscesses, liver metastases, hepatocarcinoma or cholangiocarcinoma).1–5 Histologically, they are lesions composed of a variable amount of inflammatory cells, histiocytes and myofibroblasts.1–3 Due to the absence of uniform diagnostic criteria, there are no clear data for incidence, anatomical distribution, natural history or malignant potential.3 It seems that they are more frequent in young patients, with no clear predominance of either sex.1,5 The etiology and pathogenesis are not clear, and possible causes include: germ infections (Escherichia coli, Klebsiella, bacteroides, etc.), trauma, vascular origins, and autoimmune diseases.1–5
Clinical data and imaging methods are not very specific and correct preoperative diagnosis is uncommon.2,5 IPL can be asymptomatic or cause asthenia, nonspecific abdominal pain, jaundice, intermittent fever or weight loss.1–3 They have been associated with other inflammatory lesions like sclerosing cholangitis, retroperitoneal fibrosis or autoimmune diseases.1 The lab work can be normal, or nonspecific alterations ma y be observed in the liver profile, and/or there may be elevated tumor markers (CEA and CA19-9).1–5 On ultrasound, IPL can be hypo- or hyperechoic; on CT, after the administration of contrast, the lesion can show either hypo- or hyper-uptake; on MRI, it is typically hypointense in T1 and hyperintense in T2.1–3,5 The presence of occlusive portal phlebitis with vascular collapse is frequent.2 PET is also not specific.6 Needle aspiration of IPL has a poor efficacy and is not free of risks, but it occasionally provides a correct diagnosis.1,7
If a preoperative diagnosis is reached, it has been suggested to initiate medical treatment (antibiotics, steroids or non-steroid anti-inflammatory agents).2,3,5 In addition, there have also been reports of spontaneous remission.2,5 Surgical treatment of a correctly diagnosed IPL should only be done if it is symptomatic or presents complications.1
Our case has some interesting peculiarities regarding diagnosis and histology. In patients with breast cancer, PET is the most cost-effective test for diagnosing metastatic lesions. There are very few case reports of positive PET in IPL,6 and only one case similar to ours in a patient affected by breast cancer with multiple IPL.7,8
Histologically, two diagnostic possibilities were posed: complete remission after chemotherapy ruled out by immunohistochemistry9 or IPL. The histologic profile of IPL includes a myofibroblastic tumor or IgG4 disease. Our case did not present muscle markers, and the histologic image would be compatible with IgG4 disease, but it did not meet the consensus criteria described to make the diagnosis.10 In the case of the liver, 10 IgG4-positive plasma cells per high-power field are required, or a percentage of IgG4 higher than 40%.10 There are very few case reports of IPL associated with IgG4, and these have always been in men. In these cases, some patients presented sclerosing cholangitis or IgG4 disease, such as autoimmune pancreatitis.4 Therefore, we cannot confirm that it is an IPL associated with IgG4 as it does not meet the previously proposed criteria and there was an absence of elevated serum IgG4 levels. Nonetheless, the concepts regarding this disease are changing, and in the future it may be considered IPL associated with IgG4.
Please cite this article as: Ramia JM, de la Plaza R, Perna C, Arcediano A, García-Parreño J. Seudotumor inflamatorio hepático: difícil diagnóstico preoperatorio en paciente oncológico. Cir Esp. 2015;93:201–203.