Cholangiocarcinoma is the most common malignant neoplasm of the bile duct. It is an aggressive cancer whose only potentially curative treatment is surgery. Depending on its location, cholangiocarcinoma is classified as intrahepatic, distal and perihilar or Klatskin tumor. Given the low cost-effectiveness of preoperative cytological studies, the indication for surgical treatment is usually based on clinical and radiological findings. Despite this, up to 15% of biliary strictures may be associated with other pathological processes, a fact that often cannot be corroborated until the definitive pathological study is completed1.
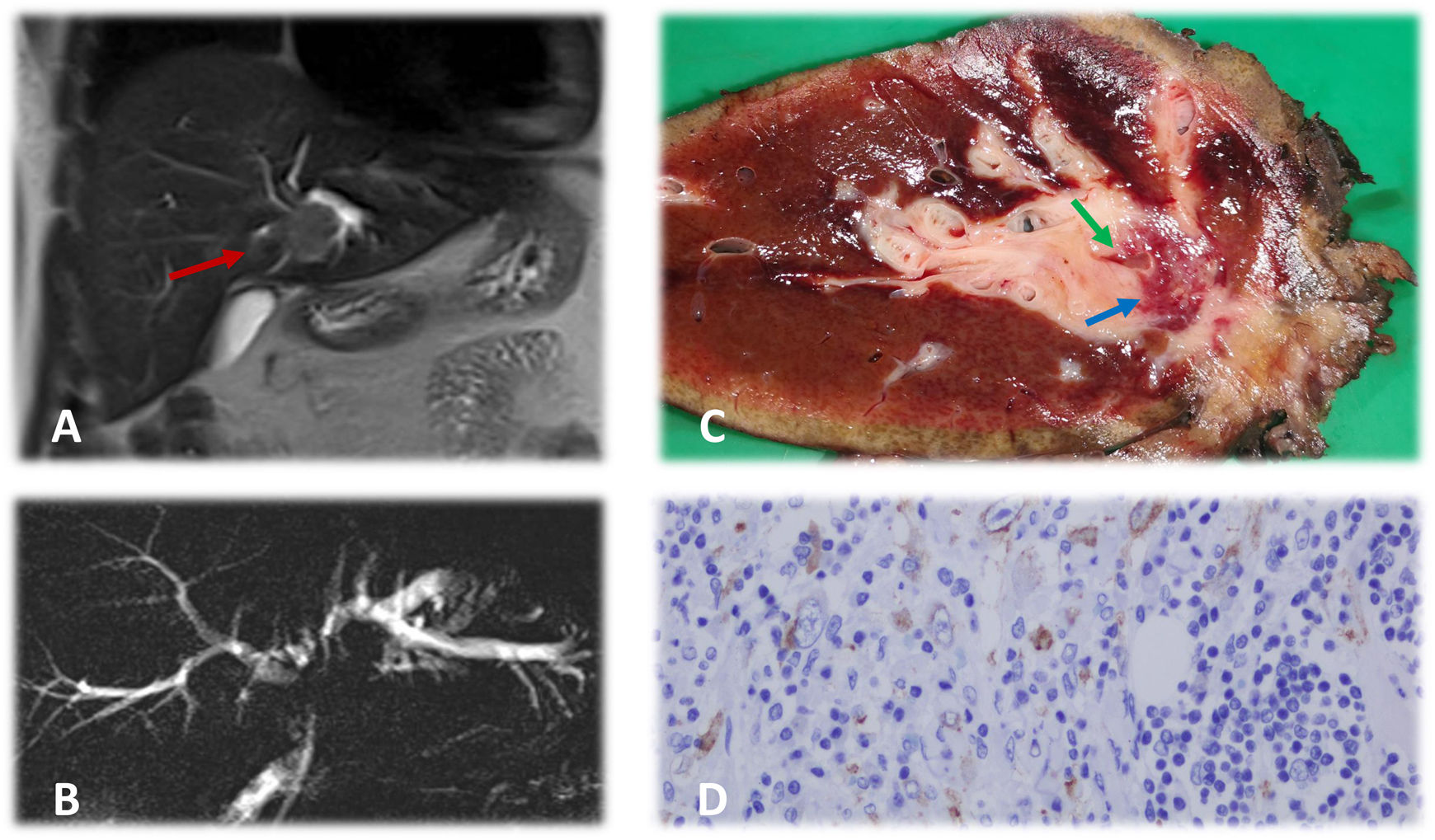
In this context, we present the case of a 37-year-old male patient who was admitted due to painless jaundice, pruritus and asthenia that had been progressing over the previous 2 weeks. His medical history of interest included a tobacco habit of 10 pack-years and laparoscopic surgery for acute appendicitis one year before the onset of the current symptoms. Lab work-up showed the following values: total bilirubin 3.8 mg/dL, direct bilirubin 3.1 mg/dL, GOT 87 U/L, GGT 356 U/L, alkaline phosphatase 227 U/L, slight leukocytosis, and negative tumor markers (CEA and CA 19.9). Imaging tests (thoracic-abdominal-pelvic CT scan and hepatic and magnetic resonance cholangiopancreatography [MRCP]) show a mass infiltrating the left hepatic duct with dilatation of the ipsilateral intrahepatic bile duct (Fig. 1, images A and B), with no distant disease. These findings suggested a type IIIb Klatskin tumor as the first possible diagnosis1. Surgical treatment was decided after evaluation by a multidisciplinary committee, involving left hepatectomy extended to the caudate lobe with resection of the main bile duct and the right hepatic duct up to the sectoral bifurcation, preserving the carina of both sectoral ducts. Biliary tract reconstruction was conducted with Roux-en-Y hepaticojejunostomy. The postoperative period was uneventful except for a low-output biliary fistula, which was resolved with conservative management.
A) Hepatic magnetic resonance study showing a mass that is neoplastic in appearance (red arrow) in the perihilar area, infiltrating the left hepatic duct and causing dilation. B) Nuclear magnetic resonance cholangiography showing notable dilation of the left intrahepatic bile duct and, to a lesser degree, of the right bile duct. Radiological ‘silence’ is observed at the bifurcation of both hepatic ducts secondary to the tumor. The common bile duct presents a normal diameter. C) Macroscopic image of the hepatectomy piece. A 2.3 × 1.9 cm lesion (blue arrow) is observed, partially encompassing the bile duct (green arrow) with a surrounding inflammatory reaction. D) Microscopic image of the hepatectomy specimen. The mass is mainly formed by a loose stroma with epithelioid cells, evident nucleoli and areas of more fasciculated distribution. Both areas present an inflammatory infiltrate comprised of lymphocytes, eosinophils, and plasma cells. Tumor cells express ALK with a characteristic nuclear membrane pattern, typical of this type of tumor.
The pathological anatomy report indicated the presence of a lesion measuring 2.3 × 1.9 cm with free margins, formed by a lax stroma and epithelioid cells with evident nucleoli, areas of fasciculated distribution and inflammatory infiltrate, which were compatible with inflammatory myofibroblastic tumor (IMT) (Fig. 1C). Tumor cells expressed ALK (anaplastic lymphoma kinase) (Fig. 1D). Other immunohistochemical markers related to rare neoplasms were negative, including CD34, CD21, CD117, CD23 and S100. With this information, we decided to complete follow-up without adjuvant treatment, and the patient was disease-free one year after the procedure.
IMT is a rare neoplasm that usually affects the lungs and abdominopelvic region. Although it has been related to other processes, such as inflammatory pseudotumor or plasma cell granuloma, it is recognized as a true soft tissue neoplasm of intermediate biological potential due to its ability to develop recurrence and metastasis2. Its appearance in the liver is rare and can lead to different forms of presentation, mimicking the behavior of other tumors. Therefore, it should be considered in the differential diagnosis of other tumors, such as hepatocarcinoma, cholangiocarcinoma, sarcomas, and benign processes like adenomas, cystic echinococcosis, or liver abscesses2–5.
IMT has been related to infectious, inflammatory and other malignant diseases2, although its etiology has yet to be clarified. It seems to be more common in young men3, a fact that differentiates it from cholangiocarcinoma, in which the age of presentation is higher (except in cases of primary sclerosing cholangitis)1. IMT does not present clinical characteristics or specific analytical markers, and elevated acute phase reactants, such as leukocytes or C-reactive protein, are only observed in some patients. Radiologically, the suspicion arises when observing a soft tissue mass with heterogeneous enhancement that may invade neighboring structures2,3. When there is involvement of the extrahepatic bile duct, diagnosis is possible by ERCP and cholangioscopy4.
As for its histology, IMT is made up of areas of proliferation of myofibroblasts mixed with collagen fibers and infiltration of inflammatory cells2. Macroscopically, it is usually solitary, with a firm consistency, well defined, not encapsulated and with a yellowish-white2,3. As a differential finding, 50%–60% of cases are positive for ALK, a factor that is related with a lower incidence of metastasis6.
Depending on the symptoms, location and suspected diagnosis, different treatment options have been described, from surgery to conservative management. The studies by Tang7 and Yang8 describe the evolution of 74 patients treated surgically for hepatic IMT, all of whom were disease-free after a mean follow-up of 30 months. Yang even reported one case of tumor regression with no treatment8. In contrast, there also are reports of cases with only a few months of survival9,10 related to RANBP2-ALK gene rearrangement, metastatic hepatic presentation after excision of the primary pelvic tumor, and advanced age. Resections with free margins and ALK positivity are related with a better prognosis3. The attached Table 1 summarizes the evolution of the patients that appear in the bibliography used.
Clinical characteristics and evolution of the patients included in the bibliography used to support the present article and in additional articles.
| Article | Age | Sex | Type of presentation | Treatment | Follow-up | Disease-free survival | Survival |
|---|---|---|---|---|---|---|---|
| Watanabe et al.2 | 70 | Female | Incidental after trauma injury | Right hepatectomy | 7 months | No recurrence | Living |
| Filips et al.3 | 32 | Female | Right hypochondrium pain and fever 4 months post-partum | Segmentectomy (IVa and IVb) | 1 year | No recurrence | Living |
| Sekaran et al.4 | 17 | Female | Melenas, jaundice and abdominal pain for 18 months | Left hepatectomy | 6 weeks | No recurrence | Living |
| Jim et al.5 | 42 | Female | Fever and fatigue for 1 month | Right posterior sectionectomy | 32 months | No recurrence | Living |
| Khalil et al.6 | 8 | Male | Liver metastasis 6 months after resection o < f IMT in the right iliac fossa | Crizotinib | 6 months | No recurrence | Living |
| Tang et al.7 | 74 | Male | Fever | Segmentectomy V | Mean follow-up 30 months | No recurrence | Living |
| 45 | Female | Fever | Segmentectomy VII | No recurrence | Living | ||
| 59 | Male | Fever and Abdominal pain | Segmentectomy IV y VIII | No recurrence | Living | ||
| 53 | Female | Abdominal pain | Segmentectomy V | No recurrence | Living | ||
| 45 | Male | Abdominal pain | Segmentectomy VI | No recurrence | Living | ||
| Chen et al.9 | 34 | Male | Weight loss and abdominal pain in the right hypochondrium | Segmentectomy V-VI | 6 months | Recurrence (5 months) | Exitus |
| Shimodaira et al.10 | 81 | Male | Pelvic IMT, treated with Miles procedure. Liver metastasis 2 months after surgery. | Surveillance | 3 months | Recurrence (2 months) | Exitus |
| Sinha et al. | 36 | Female | Inflammatory mass compromising the gall bladder, liver, pylorus and first part of the duodenum | Extended cholecystectomy with resection of the pylorus and first portion of the duodenum; Billroth II reconstruction | No | No information | No information |
| Shang et al. | 56 | Female | Liver transplantation due to cirrhosis secondary to hepatitis B virus. 5 months after transplantation, she presented melena secondary to esophageal varices and a mass was detected in the porta hepatis; the biopsy confirmed IMT. | Conservative treatment: steroids, antibiotics, antiviral agents, immunosuppression and endoscopic ligation of varices | 1 month | Recurrence (1 month) | Exitus |
| Raad et al. | 62 | Female | History of endometrial carcinoma. Follow-up imaging study detected lesion in liver segment II compatible with metastasis. The anatomopathological study after resection showed it was IMT. | Subsegmentectomy II | No | No information | No information |
| Nagarajan et al. | 1 | Male | Hepatomegaly and anemia; mass detected in left liver lobe | Initial follow-up (suspected hamartoma); after 8 months, surgical resection due to persistent symptoms and mass | 12 months | No recurrence | Living |
| Sürer et al. | 48 | Male | Pain in right hypochondrium, fever and weight loss | Surgical resection of 6-cm lesion in the right liver lobe | No | No information | Living |
| Berumen et al. | 13 | Male | Painless jaundice, asthenia; mass in hepatic hilum invading the bifurcation of both hepatic ducts, hepatic artery, portal vein, extending to the duodenum and head of pancreas. | Transhepatic biliary drainage and chemotherapy with Crizotinib; hepatotoxicity, so change to Celecoxib. | 12 months | No recurrence | Living |
| After 6 months, surgery is considered, but the mass is confirmed to be unresectable. | |||||||
| 4 months later, liver transplantation is done with pancreaticoduodenectomy. | |||||||
| Thavamani et al. | 10 | Female | Abdominal pain and fever; 5.6-cm mass in porta hepatis causing secondary portal hypertension (portal thrombosis, collateral circulation, splenomegaly) | Exploratory laparotomy, biopsy with diagnosis of IMT; treatment with Meloxicam and prednisone, with reduction of tumor size in 2 years. | 2 years | Disease persists after diagnosis | Living |
| Chablé-Montero et al. | 23 | Female | Right hypochondrium pain, fever; 7 cm mass in right liver lobe, suspected liver abscess | Antibiotic therapy; given the persistence of the lesion and symptoms, right hepatectomy is performed. | No | No information | No information |
| Schnelldorfer et al. | 18 | Female | Right hypochondrium pain, vomiting, weight loss; liver lesion causes left biliary dilatation. | Left lateral sectionectomy | 1 year | No recurrence | Living |
| Solomon et al. | 26 | Male | Right hypochondrium pain, asthenia, weight loss | Partial left hepatectomy | No | No information | No information |
Note: The series by Yang et al.8 is not included in the table because it does not provide individual data for the patients in the original article.
Additional references to those present in the bibliography of the manuscript included in the table:
- Sinha et al. International Journal of Surgery Case Reports 31 (2017) 27–29.
- Shang et al. Medicine (2017) 96:49.
- Raad et al. Clin Nucl Med 2021;46: 47–48.
- Nagarajan et al. JPGN 2013;57: 277–280.
- Sürer et al. Turk J Gastroenterol 2009; 20 (2): 129-134.
- Berumen et al. Pediatric Transplantation 2016; 1–6.
- Thavamani et al. ACG Case Rep J 2019;6:1–4.
- Chablé-Montero et al. Ann Hepatol. Sep-Oct 2012;11(5):708-9.
- Schnelldorfer et al. J Hepatobiliary Pancreat Surg (2007) 14:421–423.
- Solomon et al. Arch Pathol Lab Med (2006) 130 (10): 1548–1551.
In conclusion, whenever possible, surgical treatment seems preferable, given its intermediate biological potential. Long-term follow-up is recommended and, in case of recurrence, good response to treatment with crizotinib has been described, mainly in cases with ALK expression6.
Please cite this article as: Hinojosa Arco LC, Roldán de la Rúa JF, Arranz Salas I, Gómez Pérez R, Suárez Muñoz MÁ. Tumor miofibroblástico inflamatorio hepático que mimetiza un tumor de Klatskin. Cir Esp. 2023;101:303–307.