The term “splenosis” was used for the first time in the literature by Buchbinder and Lipkoff in 1939 to describe the implantation of splenic tissue in a heterotopic location in the case of a woman with suspected endometriosis.1 In order for this tissue implantation to occur in another part of the abdominal cavity (26%–67%), it is essential for there to have been some type of aggression on the spleen (surgery or trauma), and the probability is greater in trauma cases. The latency between the aggression and appearance of splenosis is between 5 and 10 years.2
The difference between splenosis and ectopic spleen is that the latter is congenital, more common, located close to the splenic hilum, encapsulated, and has direct vascularization from a splenic artery branch.
In descending order, the most frequent locations for splenosis are the serous surface of the small bowel, greater omentum, parietal peritoneum, large intestine, mesentery, inferior side of the diaphragm and the thorax.2 Extraperitoneal foci are rarer.
As for its radiological appearance, intrahepatic splenic tissue is usually indistinguishable from other benign or malignant lesions, and it usually behaves like an adenoma or a hepatocarcinoma.3
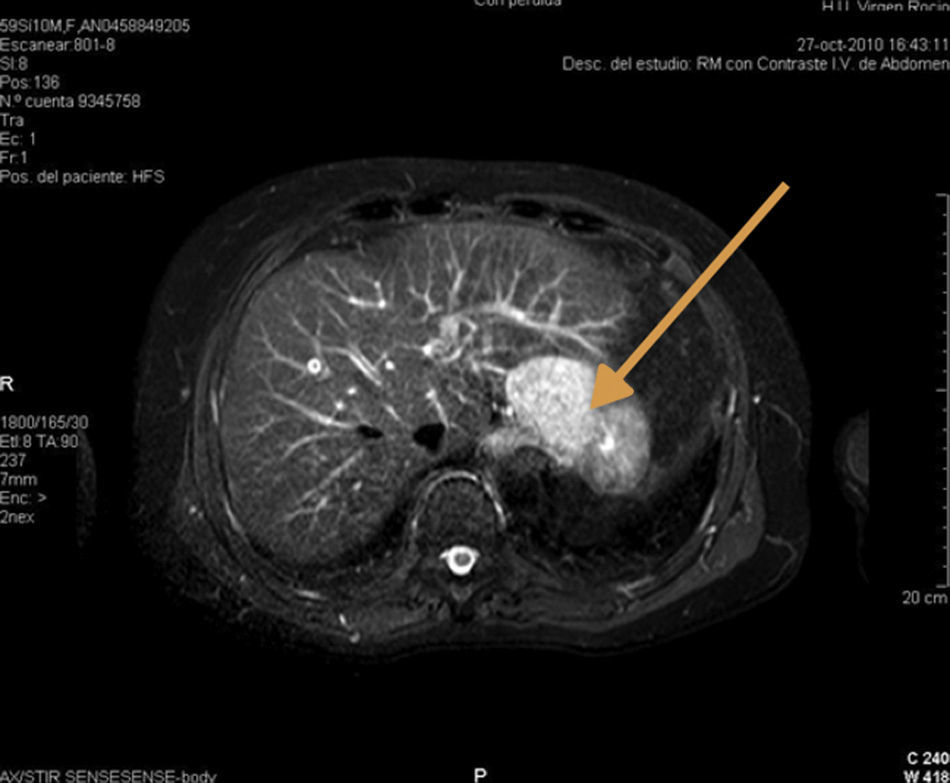
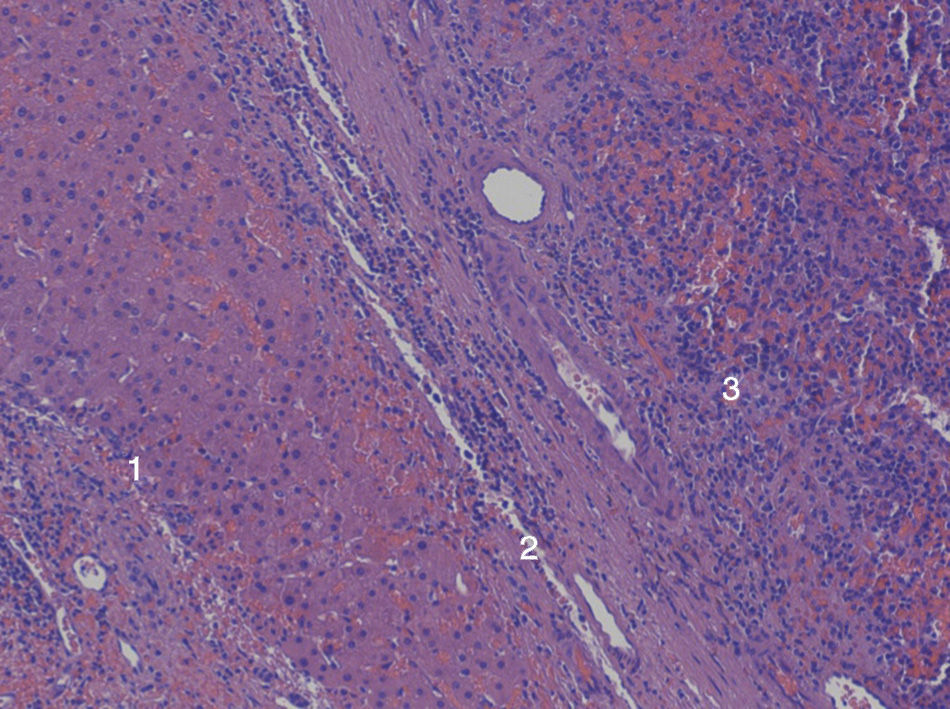
We present the case of a 60-year-old male with a prior medical history of cataracts secondary to myotonic dystrophy and recurrent peptic ulcer that had been treated surgically on 3 different occasions. During the last ulcus procedure in 1978, the spleen was injured, and emergency splenectomy was required. As a result of this event, the patient needed a red blood cell transfusion, and was diagnosed with hepatitis C infection, genotype 3a, one year later, which was treated with silymarin. In 2003, high transaminase levels were detected, and treatment with interferon and ribavirin was initiated, with a maintained viral response until 2009. In 2011, on a follow-up magnetic resonance imaging study, a focal lesion was observed in segment iii measuring 48mm (no changes respect to previous studies) that was hypervascular and showed homogenous enhancement in the arterial phase, with lavage in the portal phase and equilibrium (Fig. 1). Due to its behavior, the differential diagnosis included hepatocarcinoma and adenoma. Hepatic resection of the SOL was indicated given the absence of portal hypertension and Child-Pugh A6. During surgery, intraoperative ultrasound detected the hypoechoic lesion in segment ii,and a segmentectomy was carried out. The postoperative period was uneventful, and the patient was discharged on the sixth postoperative day. The pathology study reported the lesion to be intrahepatic splenic tissue measuring 3.5cm in diameter, surrounded by active chronic hepatitis and accompanied by moderate hepatic siderosis (grade ii) (Fig. 2).
Currently, several theories could explain intrahepatic splenosis. First of all, splenosis receives its vascularization from the surrounding tissue and has no vessels of its own, while this maintained hypoxia has been associated with poor development of the white pulp.4 Furthermore, the aging of the hepatic tissue or chronic liver disease can contribute to hyperplasia of the hepatic splenic tissue.5 Another theory defends the possibility that splenic cells that become detached during trauma reach the liver through the portal access and in this case lack any hint of a capsule.6
The presence of a capsule makes the theory of iatrogenic implantation more likely than portal migration. In the case of implantation, the edges are better defined by this capsule, which is usually 1–2mm and fibrous. In addition to this description, the liver of the patient we report presented active chronic hepatitis, which may have contributed to the development of splenosis, supporting the theory that aging or liver disease can favor this condition.3
Splenosis and accessory spleen do not usually cause symptoms but can present as abdominal pain, bowel obstruction due to adherences, gastrointestinal bleeding, torsion or spontaneous rupture. When there are symptoms, these can be confused with renal or adrenal tumors, metastatic lesions, lymphomas, endometriosis, angiomas or hepatocarcinoma.3
Macroscopically, splenosis is usually a tissue with a smooth surface that is shiny, purple-brown in color, homogeneous, and practically indistinguishable from an adenoma7 or, in cases with abundant vascularization, hepatic hemangioma. We consider that all splenectomized patients with hypervascular space-occupying masses in the arterial phase and early washout in the portal phase should be suspected of having splenosis.
For diagnosis, scintigraphic techniques offer higher diagnostic precision than CT or MRI for the diagnosis of splenosis in patients who have undergone splenectomy, especially if SPECT imaging is used.8
In general, we can conclude that scintigraphy with denatured red blood cells labeled with Tc-99m is the test of choice in cases with suspected intrahepatic splenosis.9 Moreover, if the diagnosis is confirmed, invasive measures such as surgery or percutaneous biopsy can be avoided since no malignization has been reported in this type of lesions.
Please cite this article as: Tinoco González J, Suárez Artacho G, Ramallo Solís IM, Padillo Ruiz FJ, Ángel M. Esplenosis intrahepática como diagnóstico diferencial de las lesiones hepáticas ocupantes de espacio. Cir Esp. 2014;92:690–691.