Pancreatic injury is an uncommon event often difficult to diagnose at an early stage. After abdominal trauma, the surgeon must always be aware of the possibility of pancreatic trauma due to the complications associated with missed pancreatic injuries. Due to its retroperitoneal position, associated organs and vascular injuries are almost always present, which along with frequent extra abdominal injuries explain the high morbidity and mortality. The aim of this study is to present a concise description of the incidence of these injuries, lesional mechanisms, recommended diagnostic methods, therapeutic indications including nonoperative management, endoscopy and surgery, and an analysis of pancreas-specific complications and mortality rates in these patients based on a 60-year review of the literature, encompassing 6364 patients. Due to pancreatic retroperitoneal position, associated organs and vascular injuries are almost always present, which along with frequent extraaabdominal injuries explain the high morbidity and mortality of these patients.
La lesión pancreática es un evento infrecuente y de difícil diagnóstico en fase temprana, por lo que el cirujano debe tener siempre presente esta posibilidad después de un traumatismo abdominal debido a las consecuencias que acarrean las lesiones desapercibidas. Dada su localización retroperitoneal es habitual la lesión asociada de otros órganos y estructuras vasculares abdominales, que junto con las lesiones extraabdominales explican la alta morbimortalidad de estos pacientes. El objetivo de este trabajo es presentar una descripción concisa de la incidencia de estas lesiones, los mecanismos lesionales, los métodos diagnósticos recomendados, las indicaciones de las diferentes modalidades terapéuticas conservadoras, endoscópicas y quirúrgicas disponibles, y realizar un análisis de las complicaciones específicas del páncreas y de la mortalidad en estos pacientes, basándonos en una revisión de la literatura de los últimos 60 años, habiendo identificado 6.364 pacientes. Dada la localización retroperitoneal del páncreas, es habitual la lesión asociada de otros órganos y estructuras vasculares abdominales, que junto con las lesiones extraabdominales explican la alta morbimortalidad de estos pacientes.
Pancreatic trauma is uncommon, affecting 0.5%–8% of trauma patients. The first available data were published by Travers in Lancet in 1827,2,3 where he described the findings from an autopsy.
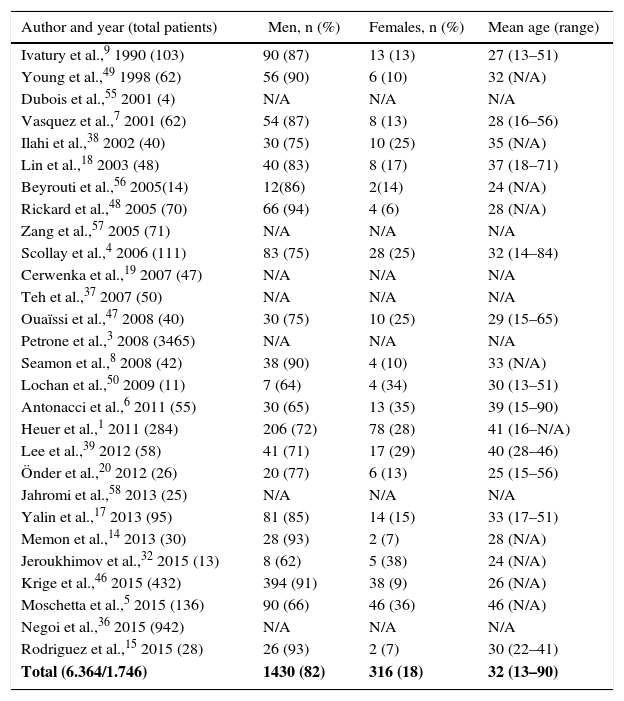
The aim of this article was to review the literature on pancreatic trauma. A search was conducted with the PubMed and Embase databases, selecting articles published in English and Spanish over the last 60 years (1956–2015). The pediatric population was excluded. A total of 28 series were identified, with a total of 6364 patients (Table 1).
Incidence by Author, Sex and Age.
| Author and year (total patients) | Men, n (%) | Females, n (%) | Mean age (range) |
|---|---|---|---|
| Ivatury et al.,9 1990 (103) | 90 (87) | 13 (13) | 27 (13–51) |
| Young et al.,49 1998 (62) | 56 (90) | 6 (10) | 32 (N/A) |
| Dubois et al.,55 2001 (4) | N/A | N/A | N/A |
| Vasquez et al.,7 2001 (62) | 54 (87) | 8 (13) | 28 (16–56) |
| Ilahi et al.,38 2002 (40) | 30 (75) | 10 (25) | 35 (N/A) |
| Lin et al.,18 2003 (48) | 40 (83) | 8 (17) | 37 (18–71) |
| Beyrouti et al.,56 2005(14) | 12(86) | 2(14) | 24 (N/A) |
| Rickard et al.,48 2005 (70) | 66 (94) | 4 (6) | 28 (N/A) |
| Zang et al.,57 2005 (71) | N/A | N/A | N/A |
| Scollay et al.,4 2006 (111) | 83 (75) | 28 (25) | 32 (14–84) |
| Cerwenka et al.,19 2007 (47) | N/A | N/A | N/A |
| Teh et al.,37 2007 (50) | N/A | N/A | N/A |
| Ouaïssi et al.,47 2008 (40) | 30 (75) | 10 (25) | 29 (15–65) |
| Petrone et al.,3 2008 (3465) | N/A | N/A | N/A |
| Seamon et al.,8 2008 (42) | 38 (90) | 4 (10) | 33 (N/A) |
| Lochan et al.,50 2009 (11) | 7 (64) | 4 (34) | 30 (13–51) |
| Antonacci et al.,6 2011 (55) | 30 (65) | 13 (35) | 39 (15–90) |
| Heuer et al.,1 2011 (284) | 206 (72) | 78 (28) | 41 (16–N/A) |
| Lee et al.,39 2012 (58) | 41 (71) | 17 (29) | 40 (28–46) |
| Önder et al.,20 2012 (26) | 20 (77) | 6 (13) | 25 (15–56) |
| Jahromi et al.,58 2013 (25) | N/A | N/A | N/A |
| Yalin et al.,17 2013 (95) | 81 (85) | 14 (15) | 33 (17–51) |
| Memon et al.,14 2013 (30) | 28 (93) | 2 (7) | 28 (N/A) |
| Jeroukhimov et al.,32 2015 (13) | 8 (62) | 5 (38) | 24 (N/A) |
| Krige et al.,46 2015 (432) | 394 (91) | 38 (9) | 26 (N/A) |
| Moschetta et al.,5 2015 (136) | 90 (66) | 46 (36) | 46 (N/A) |
| Negoi et al.,36 2015 (942) | N/A | N/A | N/A |
| Rodriguez et al.,15 2015 (28) | 26 (93) | 2 (7) | 30 (22–41) |
| Total (6.364/1.746) | 1430 (82) | 316 (18) | 32 (13–90) |
The total number of patients found was 6364; of these, patient sex was defined in only 1746.
N/A: not available.
The pancreas presents a low prevalence of traumatic injury, mainly due to its protective retroperitoneal location, which at the same time hinders and delays diagnosis. From the review of the literature, prevalence was observed to vary from 0.21% to 5.37%. In regions with a higher frequency of violent incidents, most patients were young males.1,4–9 Our study observed 82% of males, with a mean age of 32 years (Table 1).
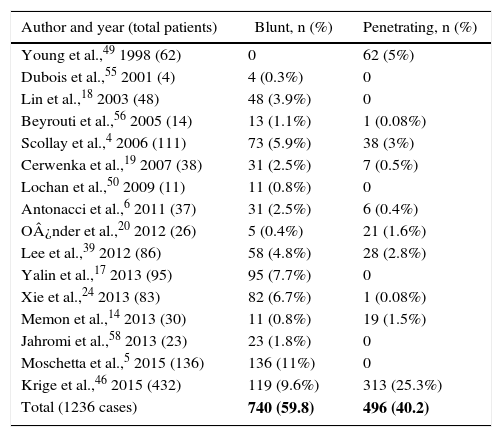
Injury MechanismThere are some publications that report a greater occurrence of pancreatic injury secondary to penetrating abdominal trauma,3,8,10–15 representing between 1% and 12% of cases,7,15–17 and about 5% blunt trauma.18,19 This distribution varies according to the origin of the series and the type of trauma. For instance, in studies published in the United Kingdom, blunt abdominal trauma is responsible for pancreatic injury in 82% of patients, whereas in studies from the United States and South Africa there is a predominance of penetrating injuries.4,14,20 In the review, it was possible to identify the mechanism of injury in 1236 cases: 59.8% were due to a blunt mechanism, and 40.2% a penetrating mechanism. Of these, 59% were caused by firearms (Table 2).
Mechanism of Pancreatic Trauma.
| Author and year (total patients) | Blunt, n (%) | Penetrating, n (%) |
|---|---|---|
| Young et al.,49 1998 (62) | 0 | 62 (5%) |
| Dubois et al.,55 2001 (4) | 4 (0.3%) | 0 |
| Lin et al.,18 2003 (48) | 48 (3.9%) | 0 |
| Beyrouti et al.,56 2005 (14) | 13 (1.1%) | 1 (0.08%) |
| Scollay et al.,4 2006 (111) | 73 (5.9%) | 38 (3%) |
| Cerwenka et al.,19 2007 (38) | 31 (2.5%) | 7 (0.5%) |
| Lochan et al.,50 2009 (11) | 11 (0.8%) | 0 |
| Antonacci et al.,6 2011 (37) | 31 (2.5%) | 6 (0.4%) |
| O¿nder et al.,20 2012 (26) | 5 (0.4%) | 21 (1.6%) |
| Lee et al.,39 2012 (86) | 58 (4.8%) | 28 (2.8%) |
| Yalin et al.,17 2013 (95) | 95 (7.7%) | 0 |
| Xie et al.,24 2013 (83) | 82 (6.7%) | 1 (0.08%) |
| Memon et al.,14 2013 (30) | 11 (0.8%) | 19 (1.5%) |
| Jahromi et al.,58 2013 (23) | 23 (1.8%) | 0 |
| Moschetta et al.,5 2015 (136) | 136 (11%) | 0 |
| Krige et al.,46 2015 (432) | 119 (9.6%) | 313 (25.3%) |
| Total (1236 cases) | 740 (59.8) | 496 (40.2) |
Blunt abdominal trauma produces a direct compression of the pancreas against the lumbar vertebrae, which, associated with the lack of displacement of the organ because of its retroperitoneal location, may cause transection of the gland in the region of the superior mesenteric vessels and even rupture of the duct.12,13,16,21 It should be noted that the review found articles referring to rare injury mechanisms.11,22–27
Associated InjuriesThe presence of associated lesions is more the rule than the exception due to location.7,11 Krige et al.10 reported the presence of isolated lesions in only 11% of cases. The incidence of isolated injuries in blunt trauma varies from 15% to 55%, whereas it is practically null when the mechanism of injury is penetrating trauma.28
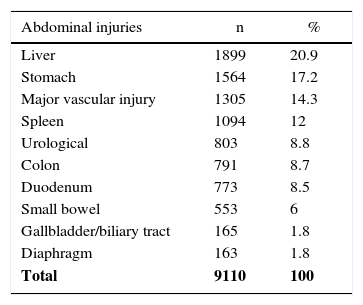
In our review, we counted 4559 patients with pancreatic injuries who presented 9110 intra-abdominal lesions (1.9 per patient). The most frequent injuries involved the liver with 1899 lesions (20.9%), followed by 1564 gastric lesions (17.2%) and 1305 major vascular lesions (14.3%) (Table 3). At the same time, 8.5% of associated duodenal lesions were identified, which increased to 12% and 21% when the mechanism of injury was penetrating trauma.7,14
Associated Abdominal and Extra-Abdominal Injuries.
| Abdominal injuries | n | % |
|---|---|---|
| Liver | 1899 | 20.9 |
| Stomach | 1564 | 17.2 |
| Major vascular injury | 1305 | 14.3 |
| Spleen | 1094 | 12 |
| Urological | 803 | 8.8 |
| Colon | 791 | 8.7 |
| Duodenum | 773 | 8.5 |
| Small bowel | 553 | 6 |
| Gallbladder/biliary tract | 165 | 1.8 |
| Diaphragm | 163 | 1.8 |
| Total | 9110 | 100 |
| Extra-abdominal injuries | n | % |
|---|---|---|
| Thorax | 234 | 44.5 |
| Musculoskeletal | 138 | 26.2 |
| Head | 92 | 17.5 |
| Neck and spinal column | 57 | 10.8 |
| Heart | 5 | 1 |
| Total (4559 injuries) | 526 | 100 |
Diagnostic suspicion is established according to the mechanism of injury and clinical presentation, although it is usually very non-specific.29,30 It is important to detect the integrity of the pancreatic duct, thereby reducing the rate of complications,31 indicating the most appropriate technique and avoiding unnecessary aggressive treatments. Imaging studies such as ultrasound or Focused Assessment Sonography for Trauma provide few or non-specific data, such as the presence of free intra-abdominal fluid, and are not useful for evaluating the entire gland.
Computed tomography (CT) is the imaging study of choice in patients with hemodynamically stable closed trauma.16 Both the sensitivity and the specificity of the test are around 85% for the detection of pancreatic injury.16 However, the sensitivity to detect duct injury is more limited (43%–54%).32,33 A disadvantage of the technique is the need to determine the ideal moment, because immediately after blunt trauma the pancreas may appear normal in 20%–40% of cases,16 and if it is performed after the first 24h the lesion may go unnoticed due to the absence of inflammatory reaction.34
If CT findings cannot define the injury, or there is a small laceration in the parenchyma, endoscopic retrograde cholangiopancreatography (ERCP) has been shown to be useful and more sensitive33 in assessing the state of the main duct. It may also reduce the severity of the injury diagnosed by CT or intraoperatively, so it can contribute to eliminate unnecessary procedures or reduce the extent of surgery.30,32,34 Another useful test for evaluating the integrity of the main pancreatic duct is magnetic resonance cholangiopancreatography (MRCP). It may also contribute to improve patient selection for conservative treatment.35 Its drawback is that it cannot be used in the initial assessment, and it is not a diagnostic option.16 One variation is dynamic magnetic resonance pancreatography after secretin stimulation; however, even though it has a high diagnostic specificity, this test is not indicated in patients with multiple injuries because of its long duration.30
Elevated secretin and amylase levels after abdominal trauma were formerly considered indicative of pancreatic injury in general. However, normal initial levels may be present in up to 35% of patients with complete ductal disconnection, which demonstrates its low sensitivity and specificity.16 It is currently considered that its elevation 3h after trauma may indicate pancreatic damage,30,36 so a series of controls is recommended.
Finally, the most invasive diagnostic option, which is mandatory in unstable patients, is surgery. During laparotomy, it is important to confirm the condition of the pancreatic duct, as the lesion may be subtle and go unnoticed. The use of intraoperative ultrasound may aid in the diagnosis of a parenchymal or even ductal lesion. In cases where the suspicion of duct injury persists, the administration of secretin can demonstrate its existence by increasing the flow of pancreatic secretion.30
Another intraoperative technique for detecting duct injury is pancreatography with cannulation of Vater's ampulla through a duodenotomy. In cases requiring distal pancreatectomy, it may be retrograde through the orifice of the main duct.3,30 The invasive nature of these 2 intraoperative techniques, as well as cholecystocholangiography with gallbladder aspiration used to demonstrate the lack of integrity of the pancreatic duct, make them unattractive and currently very rarely indicated.
According to Subramanian et al.,30 simple examination of the area of the injury for several minutes under magnification is able to demonstrate evidence of any leak of clear pancreatic fluid in most injuries involving the pancreatic duct.
Laparoscopy also plays a role in the diagnosis and treatment of pancreatic lesions, as it can be used to stage the lesion, resect distal lesions or drain proximal lesions.33
In the study by Teh et al.,37 intraoperative findings confirmed CT findings in 54.5% of cases, while confirming the presence of duct injuries in 91%. In the studies by Moschetta et al.5 and Ilahi et al.,38 lesion confirmation during surgery reached 100% of cases. Xie et al.24 also offer a positive correlation between the CT findings and laparotomy, and Lee et al.39 confirmed the presence of the lesion in 100% of the ERCP conducted.
Classification of InjuriesThe most extensively used scale for determining the degree of pancreatic injury was established by the American Association for the Surgery of Trauma (AAST)40 (Table 4).
American Association for the Surgery of Trauma (AAST) Scale for Pancreatic Injury.
| Grade | Type of injury | Description of the injury |
|---|---|---|
| I | Hematoma Laceration | Minor contusion without duct damage Superficial laceration without duct damage |
| II | Hematoma Laceration | Major contusion without duct damage or loss of tissue Major laceration without duct damage or loss of tissue |
| III | Laceration | Distal division or parenchymal damage with duct injury |
| IV | Laceration | Proximal division or parenchymal damage affecting the ampulla of Vater |
| V | Laceration | Massive disruption of the head of the pancreas |
Source: Moree et al.40
Historically, the most common trauma injuries have been grade I (60%), grade II (20%), grade III (15%) and grade IV (5%).3,28 In the review of 18 series of cases with a total of 1631 patients, in which the injury grade was specified in 1212, the most frequent pancreatic injury grade was III (275 patients, 23%), followed by grade II (196, 16%), grade I (111, 9%), grade IV (92, 8%) and grade V (35, 3%). In 503 of these patients (41.5%), the authors divided the grades into 2 categories, unifying grades I and II (304, 25%) and grades III, IV and V (199, 16%).
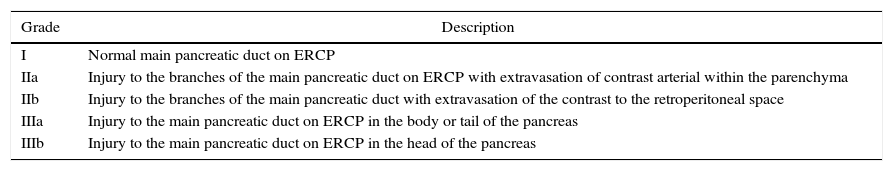
In our review, the anatomical location of the injury was only reported in 7 of these series; the body of the pancreas was the most frequent location (33.2%), followed by the tail (29.1%), head (26.8%) and neck (5.7%). In 5.2% of the remaining cases, the lesion was grouped in the neck and body. In a total of 95 patients, the degree of duct injury was reported as grade I duct damage in 15%, grade IIa 19%, IIb 23%, IIIa 32% and IIIb 11%. This gradation is defined by ERCP findings41 (Table 5).
Classification of Duct Damage by ERCP.
| Grade | Description |
|---|---|
| I | Normal main pancreatic duct on ERCP |
| IIa | Injury to the branches of the main pancreatic duct on ERCP with extravasation of contrast arterial within the parenchyma |
| IIb | Injury to the branches of the main pancreatic duct with extravasation of the contrast to the retroperitoneal space |
| IIIa | Injury to the main pancreatic duct on ERCP in the body or tail of the pancreas |
| IIIb | Injury to the main pancreatic duct on ERCP in the head of the pancreas |
ERCP: endoscopic retrograde cholangiopancreatography.
Source: Takishima et al.41
In general, grades I and II can be resolved with conservative treatment, while above grade III surgical treatment is indicated.3,16,19,28,33
Initially, the use of ERCP was only diagnostic, but recently its use has increased as a therapeutic tool both safely and satisfactorily,29,35 thereby shortening hospital stay,42 and it has been shown to be an effective option in the treatment of late-onset complications.35
Surgical treatment, however, is not only relegated to grade III or higher lesions,16,36 but also to patients who present signs of peritoneal irritation or hemodynamic instability. Factors that determine the choice of surgical technique include patient stability, injury grade, possible existence of associated lesions, integrity of the pancreatic duct, anatomical location and extension of the injury.30,42
During laparotomy, it is essential to control any active bleeding and intestinal contamination.3,30 In penetrating trauma, it may be sufficient to expose the pancreatic region of the injury pathway,30 while in blunt trauma complete exposure of the organ is necessary. The extended Kocher maneuver provides access to the anterior and posterior sides of the head and neck of the pancreas. In order to examine the anterior side of the body and tail, the gastrocolic ligament is divided and the lesser sac is accessed. With the division of the gastrohepatic ligament, the upper edge of the pancreas and the vascular elements of the splenic axis can be inspected. By releasing the adhesions below the retroperitoneum and elevating the lower edge, the back posterior side can be visualized, where the main pancreatic duct passes. For complete exposure of the tail, it is necessary to mobilize the spleen by means of blunt dissection.
The presence of saponification, clear fluid in the pancreatic bed, penetrating central lesion or deep wounds in the parenchyma are signs that indicate the high probability of injury to the pancreatic duct.43 Hematomas should be explored as they may hide a section of the duct, even if the pancreatic injuries are isolated. The main principles of treatment include hemostasis, debridement of necrotic tissue, anatomical resection and drainage.30
The most widely used surgical techniques include:External drainage: provides treatment for more than 60% of injuries.3 Closed drainage systems are preferable,30 as they are withdrawn when the discharge is minimal and the patient tolerates enteral nutrition.Pancreatography and drainage: a portion of viable greater omentum is sutured over the lesion. It is necessary to use a drain tube due to the high risk of developing a fistula.30Distal pancreatectomy: used in grades III and IV,2,10,16,30,36,44 with spleen preservation. In this trauma situation, spleen preservation is debatable as it involves a longer surgical time. The expected benefits should be considered in light of the consequences of a prolonged surgery in terms of the patient's physiological and hemodynamic reserve and age. In order to consider spleen preservation associated with distal pancreatectomy in non-elective surgery, the patient should be hemodynamically stable and normothermic, and the injury should be limited to the pancreas. The body of the pancreas should be released with ligation of the gastric vessels and completion of the pancreatic division. Placement of closed suction drains is recommended in the pancreatic bed and in the left hypochondrium. Ductal repair is determined by intraoperative findings and the extent of the injury.10,45Pancreatojejunostomy or pancreaticogastrostomy: applicable in cases of partial transection of the area of the neck or in grade IV injuries,16 with an attempt to preserve pancreatic tissue and prevent the development of pancreatic insufficiency.Roux-en-Y distal pancreaticojejunostomy: stable patients with grade IV injuries in the neck of the pancreas and few associated lesions, if any.30 The pancreatic resection is completed by ligating and dividing the residual elements and the duct is individualized for subsequent closure. A Roux-en-Y is created with a jejunal segment pulled up in a transmesocolic position to complete the anastomosis. Drains are placed at the level of the anastomosis and the stump.Pancreaticoduodenectomy: with the development of damage control surgeries, the Whipple procedure has been relegated to the last surgical option2 and is recommended in cases of massive disruption of the head of the pancreas associated with uncontrollable hemorrhage of the vena cava or portal vein, significant associated duodenal damage or destruction of the ampulla of Vater.2,16,30,44 Isolated duct damage in the head of the pancreas is not an indication to perform an emergency Whipple procedure; instead it should be drained through a duct stent.2 This should be conducted in a stepwise manner (“damage control surgery”), with an initial intervention for hemostatic control/contamination. After patient recovery, the second surgery for reconstruction is performed after 24–48h. This procedure associates important morbidity and mortality rates when performed due to trauma; mortality rates reach 30%–40%.3,16,30 Some reviews consider that only 10% of patients undergoing pancreaticoduodenectomy for pancreatic trauma actually have an indication for this procedure.16
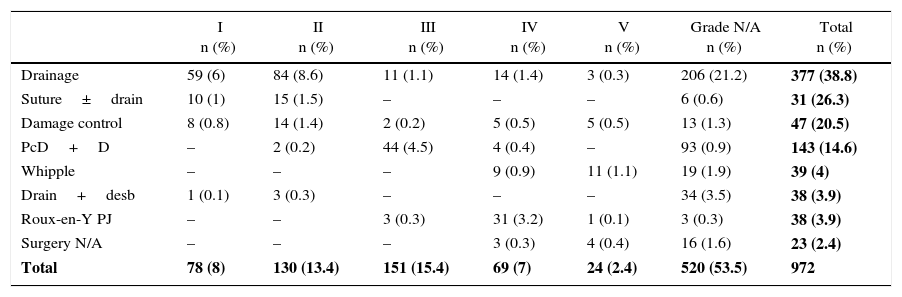
Among the series analyzed, the most frequently performed procedure was drainage in 377 (38.1%) patients, while the Whipple technique was carried out in 39 (4.1%) patients with grades IV and V injuries (Table 6).
Surgical Treatment According to AAST Pancreatic Injury Score.
| I n (%) | II n (%) | III n (%) | IV n (%) | V n (%) | Grade N/A n (%) | Total n (%) | |
|---|---|---|---|---|---|---|---|
| Drainage | 59 (6) | 84 (8.6) | 11 (1.1) | 14 (1.4) | 3 (0.3) | 206 (21.2) | 377 (38.8) |
| Suture±drain | 10 (1) | 15 (1.5) | – | – | – | 6 (0.6) | 31 (26.3) |
| Damage control | 8 (0.8) | 14 (1.4) | 2 (0.2) | 5 (0.5) | 5 (0.5) | 13 (1.3) | 47 (20.5) |
| PcD+D | – | 2 (0.2) | 44 (4.5) | 4 (0.4) | – | 93 (0.9) | 143 (14.6) |
| Whipple | – | – | – | 9 (0.9) | 11 (1.1) | 19 (1.9) | 39 (4) |
| Drain+desb | 1 (0.1) | 3 (0.3) | – | – | – | 34 (3.5) | 38 (3.9) |
| Roux-en-Y PJ | – | – | 3 (0.3) | 31 (3.2) | 1 (0.1) | 3 (0.3) | 38 (3.9) |
| Surgery N/A | – | – | – | 3 (0.3) | 4 (0.4) | 16 (1.6) | 23 (2.4) |
| Total | 78 (8) | 130 (13.4) | 151 (15.4) | 69 (7) | 24 (2.4) | 520 (53.5) | 972 |
Drain+desb: drainage and debridement; N/A: not available; PcD+S: distal pancreatectomy with splenectomy; Roux-en-Y PJ: Roux-en-Y pancreatojejunostomy; suture±drain: suture with or without drainage.
Current evidence does not recommend the use of somatostatin in trauma, and level I studies are required to justify its use. Furthermore, adequate nutritional support is crucial in this type of patients. Enteral nutrition based on a peptide formula with an elemental diet using a nasojejunal tube is ideal. Another alternative is percutaneous jejunostomy. Total parenteral nutrition is more expensive, but it can be useful if the distal enteral access is not feasible.
MorbidityThirteen series with 1009 patients7,14,15,17–20,39,46–50 reported the complications associated with pancreatic trauma and found a general rate of complications ranging from 35% to 80% and pancreas-related complications in 30%–36%. The most frequent organ-specific complications were acute pancreatitis (15%), pseudocysts (9%), abscesses (6%) and pancreatic fistulas (4%).28
According to the Krige et al.46 study of 432 consecutive cases of pancreatic trauma, the predictors for morbidity were the AAST grade and re-laparotomies. However, other groups have defined risk factors, including surgical time, complexity of surgery (bypass with poorer prognosis than resection), cardiorespiratory arrest in the operating room,17 delays in diagnosis of over 24h18,51 and inappropriate initial treatment.52
The incidence of pancreatic fistula (PF) ranges greatly due to the variability of definitions among authors. The International Study Group for Pancreatic Fistula53 proposed as a definition the abnormal communication between the pancreatic duct epithelium and another epithelial surface, and in the case of it being postoperative or after trauma, exteriorization through the drain tube. The discharge is any measurable volume after the third day post-op, with a level of amylase 3 times higher than in serum, and no need for radiological confirmation. The incidence of PF observed in our series was 11%. According to the experience of Young et al.,49 more PF occur during management with only drainage than after procedures with resection. More than 90% close in 8 weeks, and only 10% require surgery.16
Abscesses were found in 8.3%, usually in association with major duct injury. In most cases, their location was peripancreatic, subhepatic or subphrenic and could be resolved with percutaneous drainage. Intraparenchymal abscesses are uncommon and usually require surgical drainage.12
Pancreatitis can reach 18% of postoperative cases,16 and is generally mild. In case of recurrence, ERCP or MRCP12 is recommended. The evolution to necrohemorrhagic pancreatitis occurs only in 10%–15%,19,51 and its mortality rate is 80%.12,19
Long-term complications include pseudocysts and pancreatic duct stenosis. Pseudocysts occur in 5.6% of the patients analyzed. The treatment is endoscopic with ERCP when it originates in the main duct. However, injuries to the secondary branches resolve spontaneously or by percutaneous drainage. Only 3 studies reported duct stenosis: Lin et al.18 9%, Ouaïssi et al.39 2% and Krige et al.52 0.2%.
Other complications, although infrequent, include secondary bleeding from the pancreas or surrounding vessels due to retroperitoneal autodigestion, pancreatic insufficiency after resective surgery and pseudoaneurysms, mainly of the splenic artery.19
MortalityAfter analyzing 14 series and 1354 patients, the observed mortality rate was 18%, which is in agreement with data from the literature (10%–30%).1,4,17 In the articles analyzed, factors for a poor prognosis included advanced age, hemodynamic instability, blunt trauma and associated injuries.36 Other influences include the degree of pancreatic duct injury, infra-staging of the lesions, and delayed diagnosis.18,19
According to Heuer et al.,1 more than 70% of deaths after pancreatic trauma occur in the first 24h, due to large vessel injuries or severe lesions of adjacent organs.20 In fact, patients with hemodynamic shock on admission have a mortality rate of 35%, which drops to 3.7% if they arrive in stable condition.46 Many studies exclude deaths in the first 24h or prior to surgery, which could explain the great variability seen in published mortality rates. The publication by Scollay et al.4 reports the highest mortality rate (46%), including patients diagnosed by autopsy after death in the emergency room. When these cases were excluded, the remaining mortality rate was 34%, which is comparable to reports by other groups.
The mortality rate related with pancreatic injury is low. Out of the 6 series7,14,20,39,47,48 that defined this mortality subgroup, pancreatic trauma represents only 11% of the total mortality. Late mortality is also not attributed to pancreatic injury, as it is secondary to pancreatic duct injury20 with development of intra-abdominal sepsis and multiple organ failure.
A German multicenter study1 has found a 10% decrease in mortality since 2000, which is attributed to improved prehospital care. On the other hand, the introduction in the last 2 decades of the concept of damage control surgery has improved the survival of the most severe patients.15 Seamon et al.8 retrospectively studied 42 patients undergoing damage control surgery after pancreatic trauma, concluding that the presence of shock or major vascular injury dictates the extension of the surgery and results in lower mortality when shorter interventions are performed based on packing and drainage.
ConclusionsIn cases of suspected pancreatic injury, the major pancreatic duct should be evaluated meticulously since the main predictor of morbidity and mortality is duct damage,18,36,50 which will define the treatment to be followed:
- -
Non-surgical treatment is recommended when duct injury has been ruled out by CT, ERCP,30 or MRCP, with AAST grade I and III injuries secondary to blunt trauma36 and in patients with hemodynamic stability.
- -
In grades IV and V lesions with no damage to the duodenum or the ampulla of Vater, drain placement in recommended.36
- -
In hemodynamically unstable patients, damage control surgery is recommended16 because quick control of the hemorrhage and recovery from hemodynamic shock is essential to reduce high immediate mortality.46
- -
In non-bleeding pancreatic lesions with important associated injuries, one therapeutic option would be the placement of a closed suction drain in the lesser sac.2
- -
If there is duct injury or late-onset complications, the placement of a transpapillary stent by means of ERCP or a nasopancreatic drain tube favors healing, especially if the placement is done in early stages,30 interrupting the leak point and blocking the discharge of pancreatic juices, or with the conversion of a high-low pressure system in the duct by opening the sphincter.35
- -
In the case of pseudocysts, currently the technique of choice is pseudocyst-gastric drainage using endoscopic ultrasound. It has shown greater efficacy, especially in small lesions or with a poor window, and even when changing the therapeutic approach in more than one-third of cases.52 Simple radio-guided transabdominal drainage is a useful alternative.
- -
The presence of pseudoaneurysms should be ruled out in patients with necrotizing pancreatitis to thereby prevent bleeding due to their rupture. If any are found, they should be angioembolized.
The authors have no conflict of interests to declare.
Please cite this article as: Petrone P, Moral Álvarez S, González Pérez M, Ceballos Esparragón J, Marini CP. Traumatismos de páncreas: manejo y revisión de la literatura. Cir Esp. 2017;95:123–130.